Asymmetric Counteranion-Directed Halogen Bonding Catalysis
- PMID: 40029961
- PMCID: PMC11912313
- DOI: 10.1021/jacs.4c18378
Asymmetric Counteranion-Directed Halogen Bonding Catalysis
Abstract
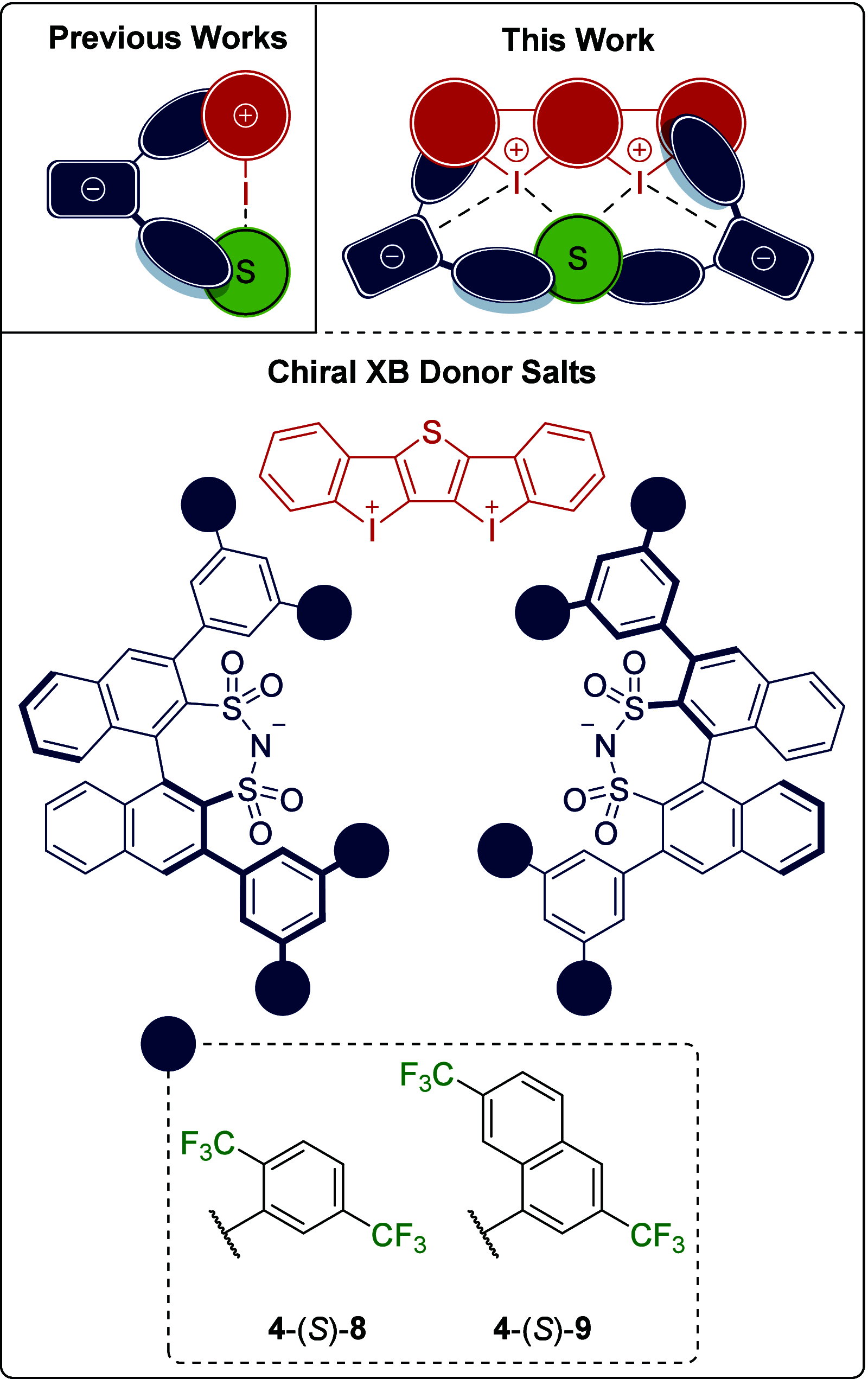
Halogen bonding has been established as a promising tool in organocatalysis. Asymmetric processes are nevertheless scarce, and their applications are limited to a few studies applying chiral halogen bond donors. Herein, we combine halogen bonding with asymmetric counteranion-directed catalysis, providing the first highly enantioselective example of such an approach. A strong bidentate iodine(III)-based catalyst with chiral disulfonimides as counteranions is applied in the first asymmetric organocatalysis of the Diels-Alder reaction between cyclopentadiene and trans-β-nitrostyrene, the key step in the synthesis of the drug fencamfamine, which was prepared with high enantioselectivity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
LinkOut - more resources
Full Text Sources

