Interim PET after 4 cycles predicts outcome in histomolecularly confirmed primary mediastinal B-cell lymphoma
- PMID: 40030008
- PMCID: PMC12088757
- DOI: 10.1182/bloodadvances.2024015577
Interim PET after 4 cycles predicts outcome in histomolecularly confirmed primary mediastinal B-cell lymphoma
Abstract
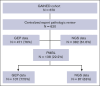
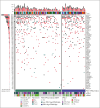
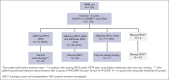
The GAINED study was a randomized phase 3 trial comparing obinutuzumab (G) with rituximab (R) plus ACVBP (doxorubicin, cyclophosphamide, and prednisone, combined with either vindesine or bleomycin) or CHOP14 (cyclophosphamide, doxorubicin, vincristine, and prednisone, administered on a 14-day schedule) induction, followed by positron emission tomography (PET)-guided consolidation. This post hoc analysis aimed to detail the outcomes of patients with primary mediastinal B-cell lymphoma (PMBL), verified through expert pathological review and the use of gene expression profiling (GEP) and next-generation sequencing. Of 620 centrally reviewed patients, 138 (22.3%) confirmed PMBL cases were analyzed. Baseline characteristics included a median age of 33.5 years, 63.8% female, 55.1% stage III to IV, 90.6% elevated lactate dehydrogenase, 87.6% Eastern Cooperative Oncology Group performance status score of 0 to 1, 62.3% extranodal involvement, 52.6% age-adjusted International Prognostic Index (aaIPI) of 2% to 3%, and 53.6% bulk (>10 cm). Induction regimens were R/G-CHOP14 (56.9%) and R/G-ACVBP (43.1%). Postinduction treatments, based on interim PET results, included: standard consolidation chemotherapy (59.8%) if change in maximum standardized uptake value (ΔSUVmax) of >66% after cycle 2 and >70% after cycle 4 (PET2-/4-), intensive treatment and autologous transplantation (26.8%) if PET2+/4-, and salvage therapy (13.4%) if PET4+ (ΔSUVmax of ≤70%). Among patients with GEP data (n = 107), 38 (35.5%) were PDL1high/PDL2high. Key somatic mutations data (n = 87) included SOCS1 (70.1%), B2M (56.3%), STAT6 (49.4%), TNFAIP3 (47.1%), GNA13 (39.1%), CIITA (37.9%), CD58 (36.8%), and TP53 (29.9%). After a median follow-up of 39.5 months, 2-year progression-free survival (PFS) and overall survival (OS) rates were 86.2% and 93.2%, respectively. In a multivariate model including bulk, aaIPI, and ΔSUVmax PET2/PET4, only bulk and ΔSUVmax PET4 of ≤70% were associated with shorter PFS (hazard ratio, 4.39 [95% confidence interval (CI), 1.28-15.11] and 4.95 [95% CI, 1.71-14.3], respectively), whereas none were associated with OS. The ΔSUVmax-based interim PET4 response emerged as the strongest predictor of patient outcomes in this selected clinical trial population. This trial was registered at www.ClinicalTrials.gov as #NCT01659099.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: V.C. has received honoraria from AbbVie, AstraZeneca, Bristol Myers Squibb (BMS), Ideogen, Janssen, Kiowa Kirin, Kite/Gilead, Novartis, Pfizer, Sanofi, and Takeda. R.H. has received honoraria from Kite/Gilead, Novartis, BMS/Celgene, Incyte, Janssen, Merck Sharp & Dohme., Takeda, Amgen, AbbVie, and Roche; and is a member on an entity’s board of directors or advisory committees of Kite/Gilead, Novartis, BMS/Celgene, Tessa Therapeutics, AbbVie, and Roche. W.B. has received travel grants from Leo Pharma, Roche, and Takeda; and honoraria from Leo Pharma and BMS. The remaining authors declare no competing financial interests.
Figures



Similar articles
-
FDG-PET-driven consolidation strategy in diffuse large B-cell lymphoma: final results of a randomized phase 2 study.Blood. 2017 Sep 14;130(11):1315-1326. doi: 10.1182/blood-2017-02-766691. Epub 2017 Jul 12. Blood. 2017. PMID: 28701367 Clinical Trial.
-
Obinutuzumab vs rituximab for advanced DLBCL: a PET-guided and randomized phase 3 study by LYSA.Blood. 2021 Apr 29;137(17):2307-2320. doi: 10.1182/blood.2020008750. Blood. 2021. PMID: 33211799
-
Favorable outcome of primary mediastinal large B-cell lymphoma patients treated with sequential RCHOP-RICE regimen without radiotherapy.Cancer Chemother Pharmacol. 2016 May;77(5):1053-60. doi: 10.1007/s00280-016-3024-8. Epub 2016 Apr 7. Cancer Chemother Pharmacol. 2016. PMID: 27056383
-
PET-guided omission of radiotherapy in early-stage unfavourable Hodgkin lymphoma (GHSG HD17): a multicentre, open-label, randomised, phase 3 trial.Lancet Oncol. 2021 Feb;22(2):223-234. doi: 10.1016/S1470-2045(20)30601-X. Lancet Oncol. 2021. PMID: 33539742 Clinical Trial.
-
Training improves the interobserver agreement of the expert positron emission tomography review panel in primary mediastinal B-cell lymphoma: interim analysis in the ongoing International Extranodal Lymphoma Study Group-37 study.Hematol Oncol. 2017 Dec;35(4):548-553. doi: 10.1002/hon.2339. Epub 2016 Aug 22. Hematol Oncol. 2017. PMID: 27545416 Review.
References
-
- Lekovic D, Miljic P, Mihaljevic B. Increased risk of venous thromboembolism in patients with primary mediastinal large B-cell lymphoma. Thromb Res. 2010;126(6):477–480. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

