In the activation of HPV-specific human B cells HPV-VLP vaccines mimic membrane-associated antigens
- PMID: 40030014
- PMCID: PMC11912367
- DOI: 10.1073/pnas.2414514122
In the activation of HPV-specific human B cells HPV-VLP vaccines mimic membrane-associated antigens
Abstract
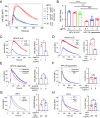
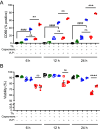
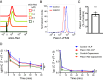
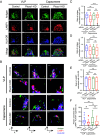
B cell responses to membrane-presented antigens appear to be strongly favored over soluble antigens in vivo suggesting that vaccines that mimic membrane-presented antigens may be highly efficacious. We recently demonstrated that human B cell responses to membrane-associated but not to soluble antigens in vitro depended on the expression and activity of the plasma membrane mechanosensitive ion channel, Piezo1. Here, we provide evidence that the efficacy of the current human papillomavirus virus-like particle (HPV VLP) vaccines may be due in part to their inherent ability to mimic Piezo1-dependent membrane presentation of antigens to B cells. We compared HPV-specific human B cell responses to HPV VLPs versus soluble HPV pentameric capsomeres and showed that although both induced calcium responses, only HPV VLP-induced responses were blocked by Piezo1 inhibitors. The kinetics of internalization of HPV-VLP and capsomeres into HPV-specific B cells were similar and neither required Piezo1 function as shown by small interfering RNA (siRNA)-mediated knockdown of Piezo. However, trafficking of HPV-VLPs into intracellular major histocompatibility complex (MHC) class II, lysosomal associated membrane protein 1 (LAMP1)+ antigen-processing compartments was Piezo1-dependent, whereas trafficking of capsomeres was not. In addition, a time course of intracellular trafficking suggested that colocalization of HPV-VLP with MHC classII was more stable over time as compared to capsomeres. Taken together these findings suggest that the ability of HPV-VLP vaccines to mimic Piezo1-dependent membrane antigen presentation may be exploited in the design of highly effective human vaccines.
Keywords: HPV-VLP vaccine; Piezo1; antigen presentation; human B cells.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

References
-
- Bachmann M. F., et al. , The influence of antigen organization on B cell responsiveness. Science 262, 1448–1451 (1993). - PubMed
-
- Batista F. D., Neuberger M. S., Affinity dependence of the B cell response to antigen: A threshold, a ceiling, and the importance of off-rate. Immunity 8, 751–759 (1998). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

