TEX38 localizes ZDHHC19 to the plasma membrane and regulates sperm head morphogenesis in mice
- PMID: 40030029
- PMCID: PMC11912386
- DOI: 10.1073/pnas.2417943122
TEX38 localizes ZDHHC19 to the plasma membrane and regulates sperm head morphogenesis in mice
Abstract
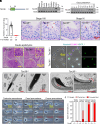
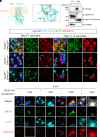
Sperm morphogenesis is a tightly regulated differentiation process, disruption of which leads to sperm malfunction and male infertility. Here, we show that Tex38 knockout (KO) male mice are infertile. Tex38 KO spermatids exhibit excess retention of residual cytoplasm around the head, resulting in abnormal sperm morphology with backward head bending. TEX38 interacts and colocalizes with ZDHHC19, a testis-enriched acyltransferase catalyzing protein S-palmitoylation, at the plasma membrane of spermatids. ZDHHC19 and TEX38 are each downregulated in mouse testes lacking the other protein. TEX38 stabilizes and localizes ZDHHC19 to the plasma membrane of cultured cells and vice versa, consolidating their interdependence. Mice deficient in ZDHHC19 or harboring a C142S mutation that disables the palmitoyltransferase activity of ZDHHC19 display phenotypes resembling those of Tex38 KO mice. Strikingly, ZDHHC19 palmitoylates ARRDC5, an arrestin family protein regulating sperm differentiation. Overall, our findings indicate that TEX38 forms a stable complex with ZDHHC19 at the plasma membrane of spermatids, which governs downstream S-palmitoylation of proteins essential for morphological transformation of spermatids.
Keywords: S-acylation; spermiation; sterility.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Miyata H., Shimada K., Kaneda Y., Ikawa M., Development of functional spermatozoa in mammalian spermiogenesis. Development 151, dev202838 (2024). - PubMed
-
- Clermont Y., Kinetics of spermatogenesis in mammals: Seminiferous epithelium cycle and spermatogonial renewal. Physiol. Rev. 52, 198–236 (1972). - PubMed
-
- Breucker H., Schäfer E., Holstein A.-F., Morphogenesis and fate of the residual body in human spermiogenesis. Cell Tissue Res. 240, 303–309 (1985). - PubMed
MeSH terms
Substances
Grants and funding
- JP21H05033/MEXT | Japan Society for the Promotion of Science (JSPS)
- P01 HD087157/HD/NICHD NIH HHS/United States
- NA/Takeda Science Foundation (TSF)
- JP23KJ1523/MEXT | Japan Society for the Promotion of Science (JSPS)
- JPMJFR211F/MEXT | JST | Fusion Oriented REsearch for disruptive Science and Technology (FOREST)
- JP23K18328/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP24K09314/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP21H04753/MEXT | Japan Society for the Promotion of Science (JSPS)
- R01HD088412/HHS | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- JP19H05750/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP24K02033/MEXT | Japan Society for the Promotion of Science (JSPS)
- R01 HD088412/HD/NICHD NIH HHS/United States
- JP22H03214/MEXT | Japan Society for the Promotion of Science (JSPS)
- P01HD087157/HHS | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- JP23K05831/MEXT | Japan Society for the Promotion of Science (JSPS)
- INV-001902/GATES/Gates Foundation/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

