Specific microbial ratio in the gut microbiome is associated with multiple sclerosis
- PMID: 40030030
- PMCID: PMC11912405
- DOI: 10.1073/pnas.2413953122
Specific microbial ratio in the gut microbiome is associated with multiple sclerosis
Abstract
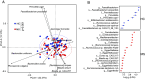
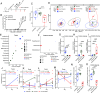
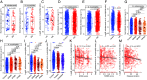
Gut microbiota dysbiosis is associated with multiple sclerosis (MS), but the causal relationship between specific gut bacteria and MS pathogenesis remains poorly understood. Therefore, we profiled the stool microbiome of people with MS (PwMS) and healthy controls (HC) using shotgun metagenomic sequencing. PwMS showed a distinct microbiome compared to HC, with Prevotella copri (PC) and Blautia species as drivers of microbial communities in HC and PwMS, respectively. Administration of MS-driving Blautia species (Blautia wexlerae; BW) to mice resulted in increased levels of gut inflammatory markers and altered microbiota with increased capacity to induce proinflammatory cytokines. Utilizing experimental autoimmune encephalomyelitis (EAE), an animal model of MS, we identified a lower gut Bifidobacterium to Akkermansia ratio as a hallmark of the disease. BW-administered mice also showed a lower Bifidobacterium to Akkermansia ratio pre-EAE induction which correlated with increased disease severity post-EAE induction. The importance of the Bifidobacterium to Akkermansia ratio at the species level, lower Bifidobacterium adolescentis to Akkermansia muciniphila (BA:AM), was validated in our MS cohort and a large International Multiple Sclerosis Microbiome Study. Thus, our findings highlight the BA:AM ratio as a potential gut microbial marker in PwMS, opening avenues for microbiome-based diagnosis, prognosis, and therapy in MS.
Keywords: gut microbial markers; gut microbiome; inflammation; microbial ratio; multiple sclerosis.
Conflict of interest statement
Competing interests statement:A.K.M. holds a patent (licensed to Evelo Biosciences) on a technology using
Figures




References
-
- Ebers G. C., et al. , A population-based study of multiple sclerosis in twins. N. Engl. J. Med. 315, 1638–1642 (1986). - PubMed
-
- Neufeld K. M., Kang N., Bienenstock J., Foster J. A., Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 23, 255–264.e119 (2011). - PubMed
-
- Iwai H., Ishihara Y., Yamanaka J., Ito T., Effects of bacterial flora on cecal size and transit rate of intestinal contents in mice. Jpn J. Exp. Med. 43, 297–305 (1973). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

