Dynamic investigation of hypoxia-induced L-lactylation
- PMID: 40030031
- PMCID: PMC11912421
- DOI: 10.1073/pnas.2404899122
Dynamic investigation of hypoxia-induced L-lactylation
Abstract
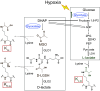
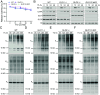
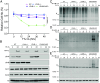
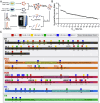
The recently identified histone modification lysine lactylation can be stimulated by L-lactate and glycolysis. Although the chemical group added upon lysine lactylation was originally proposed to be the L-enantiomer of lactate (KL-la), two isomeric modifications, lysine D-lactylation (KD-la) and N-ε-(carboxyethyl) lysine (Kce), also exist in cells, with their precursors being metabolites of glycolysis. The dynamic regulation and differences among these three modifications in response to hypoxia remain poorly understood. In this study, we demonstrate that intracellular KL-la, but not KD-la or Kce, is up-regulated in response to hypoxia. Depletion of glyoxalase enzymes, GLO1 and GLO2, had minimal impact on KD-la, Kce, or hypoxia-induced KL-la. Conversely, blocking glycolytic flux to L-lactate under hypoxic conditions by knocking out lactate dehydrogenase A/B completely abolished the induction of KL-la but increased KD-la and Kce. We further observed a correlation between the level of KL-la and hypoxia-inducible factor 1 alpha (HIF-1α) expression under hypoxic conditions and when small molecules were used to stabilize HIF-1α in the normoxia condition. Our result demonstrated that there is a strong correlation between HIF-1α and KL-la in lung cancer tissues and that patient samples with higher grade tend to have higher KL-la levels. Using a proteomics approach, we quantified 66 KL-la sites that were up-regulated by hypoxia and demonstrated that p300/CBP contributes to hypoxia-induced KL-la. Collectively, our study demonstrates that KL-la, rather than KD-la or Kce, is the prevailing lysine lactylation in response to hypoxia. Our results therefore demonstrate a link between KL-la and the hypoxia-induced adaptation of tumor cells.
Keywords: LC–MS/MS; hypoxia; lactylation; posttranslational modification (PTM).
Conflict of interest statement
Competing interests statement:Y.Z. is a founder, board member, advisor to, and inventor on patents licensed to PTM Bio Inc. (Hangzhou, China and Chicago, IL) and Maponos Therapeutics Inc. (Chicago, IL). L.B. is a co-founder, board member, advisor to, and inventor on patents licensed to Onchilles Pharma Inc.; a co-founder, board member, and inventor on patents licensed to MacroLogic Inc.; and a co-founder and inventor on patents licensed to Maponos Therapeutics Inc. K.C. is an inventor on a patent licensed to MacroLogic Inc. The other authors declare no competing interests.
Figures


References
-
- Certo M., Tsai C. H., Pucino V., Ho P. C., Mauro C., Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat. Rev. Immunol. 21, 151–161 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

