Host Immune Cell Membrane Deformability Governs the Uptake Route of Malaria-Derived Extracellular Vesicles
- PMID: 40030053
- PMCID: PMC11924330
- DOI: 10.1021/acsnano.4c07503
Host Immune Cell Membrane Deformability Governs the Uptake Route of Malaria-Derived Extracellular Vesicles
Abstract
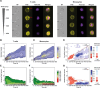
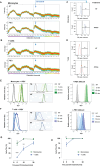
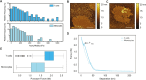
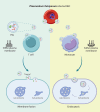
The malaria parasite, Plasmodium falciparum, secretes extracellular vesicles (EVs) to facilitate its growth and to communicate with the external microenvironment, primarily targeting the host's immune cells. How parasitic EVs enter specific immune cell types within the highly heterogeneous pool of immune cells remains largely unknown. Using a combination of imaging flow cytometry and advanced fluorescence analysis, we demonstrated that the route of uptake of parasite-derived EVs differs markedly between host T cells and monocytes. T cells, which are components of the adaptive immune system, internalize parasite-derived EVs mainly through an interaction with the plasma membrane, whereas monocytes, which function in the innate immune system, take up these EVs via endocytosis. The membranal/endocytic balance of EV internalization is driven mostly by the amount of endocytic incorporation. Integrating atomic force microscopy with fluorescence data analysis revealed that internalization depends on the biophysical properties of the cell membrane rather than solely on molecular interactions. In support of this, altering the cholesterol content in the cell membrane tilted the balance in favor of one uptake route over another. Our results provide mechanistic insights into how P. falciparum-derived EVs enter into diverse host cells. This study highlights the sophisticated cell-communication tactics used by the malaria parasite.
Keywords: EVs; cellular uptake; extracellular vesicles; imaging flow cytometry; malaria; membrane deformability.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- World Malaria Report 2023. https://www.who.int/teams/global-malaria-programme/reports/world-malaria.... (accessed April 18, 2024).
-
- Sisquella X.; Ofir-Birin Y.; Pimentel M. A.; Cheng L.; Abou Karam P.; Sampaio N. G.; Penington J. S.; Connolly D.; Giladi T.; Scicluna B. J.; Sharples R. A.; Waltmann A.; Avni D.; Schwartz E.; Schofield L.; Porat Z.; Hansen D. S.; Papenfuss A. T.; Eriksson E. M.; Gerlic M.; Hill A. F.; Bowie A. G.; Regev-Rudzki N. Malaria Parasite DNA-Harbouring Vesicles Activate Cytosolic Immune Sensors. Nat. Commun. 2017, 8 (1), 1985.10.1038/s41467-017-02083-1. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

