Infantile TK2 Deficiency Causing Mitochondrial Encephalomyopathy With Migrating Focal Seizures
- PMID: 40030095
- PMCID: PMC11879469
- DOI: 10.1212/WNL.0000000000213373
Infantile TK2 Deficiency Causing Mitochondrial Encephalomyopathy With Migrating Focal Seizures
Abstract
Objective: Recessive variants in the TK2 gene cause thymidine kinase 2 deficiency (TK2d) presenting with infantile, childhood, or adult-onset myopathy. CNS involvement is reported in only 25% of the infantile form. Compassionate use of deoxynucleoside substrate enhancement therapy (dC/dT) has been demonstrated safe and effective in TK2d myopathy, but no data are available on the potential efficacy on the human brain disease.
Methods: Here, we report for the first time a patient with infantile TK2d epileptic encephalomyopathy enrolled in an early access program with dC/dT treatment (MT1621).
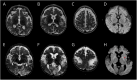
Results: At age 3 months, he presented progressive hypotonia, motor regression, failure to thrive, and respiratory failure. At age 8 months, he developed drug-resistant epilepsy with migrating focal seizures. Brain MRI showed progressive atrophy and bilateral subcortical lesions with lactate peak. Exome sequencing revealed 2 novel biallelic heterozygous variants in the TK2 gene (c.182G>A, p.Ser61Asn, c.704 T>C, p.Ile235Thr) whose pathogenicity was confirmed with in vitro studies. Early access compassionate use of dC/dT at 400 mg/kg prolonged the survival and stabilized the muscle disease but was not effective on the brain.
Discussion: Our report highlights the importance of deep-phenotyping infantile TK2d before dC/dT supplementation to stratify disease severity further and suggests a limited tissue-specific brain efficacy.
Conflict of interest statement
L. Bergonzini, S. Carli, S. Pelle, I. Pettenuzzo, S. Bonetti, E. Santi, C. Visconti, M. Maffei, M. Sheremet, E. Lamantea, A. Marsala, O. Klub, V. Gentile, D.M. Cordelli report no disclosures relevant to the manuscript. C. Garone is a paid consultant to UCB Pharma under a scientific advisory consultancy contract countersigned by the University of Bologna. C. Garone is also a scientist with a Columbia University patent for dC/dT treatment, which is licensed by Modis Therapeutics. Go to
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
