HER2-Selective Tyrosine Kinase Inhibitor, Zongertinib (BI 1810631), in Patients With Advanced/Metastatic Solid Tumors With HER2 Alterations: A Phase Ia Dose-Escalation Study
- PMID: 40030100
- PMCID: PMC11974636
- DOI: 10.1200/JCO-24-01727
HER2-Selective Tyrosine Kinase Inhibitor, Zongertinib (BI 1810631), in Patients With Advanced/Metastatic Solid Tumors With HER2 Alterations: A Phase Ia Dose-Escalation Study
Abstract
Purpose: Human epidermal growth factor receptor 2 (HER2) alterations occur in many solid cancers, including non-small cell lung cancer (NSCLC). Beamion LUNG-1 (ClinicalTrials.gov identifier: NCT04886804) is assessing the safety/efficacy of zongertinib (BI 1810631), a novel HER2-selective tyrosine kinase inhibitor that spares epidermal growth factor receptor, in patients with HER2-altered solid tumors.
Materials and methods: Beamion LUNG-1 is an ongoing multicenter, multicohort phase Ia/Ib trial. Phase Ia assessed zongertinib administered twice a day (15-150 mg) or once daily (60-360 mg) in pretreated patients with various tumors, including NSCLC. Primary end points were maximum tolerated dose (MTD) and dose-limiting toxicities (DLTs); tumor response was a secondary end point.
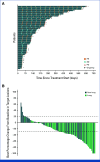
Results: As of May 23, 2024, 105 patients were treated. Two DLTs occurred during the MTD evaluation period; MTD was not reached (NR). The recommended doses for expansion were 120 mg once daily and 240 mg once daily. Treatment-related adverse events (TRAEs; any/grade ≥3) occurred in 82%/10% of patients. The most common TRAEs (any/grade ≥3) included diarrhea (50%/1%), rash (16%/2%), anemia (10%/0%), decreased appetite (10%/1%), and increased alanine transaminase (10%/4%). The confirmed investigator-assessed overall response rate (ORR) across all doses/tumors was 30% (95% CI, 23 to 40); median duration of response was 12.7 months (95% CI, 6.9 to NR). In 54 patients with NSCLC, confirmed ORR was 35% (95% CI, 24 to 49). Activity was observed in patients with A775_G776insYVMA (ORR, 38%) and those who had received previous HER2-directed therapy (ORR, 28%). In patients with NSCLC receiving zongertinib once daily, median progression-free survival was 17.2 months (95% CI, 8.3 to NR).
Conclusion: Zongertinib had a manageable safety profile and demonstrated preliminary antitumor activity in patients with HER2-altered tumors, including those with HER2-mutant NSCLC.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
References
-
- US Food and Drug Administration : ENHERTU® (fam-trastuzumab deruxtecan-nxki) highlights of prescribing information, 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761139s028lbl.pdf
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

