Pembrolizumab in gestational trophoblastic neoplasia: Systematic review and meta-analysis with sub-group analysis of potential prognostic factors
- PMID: 40031424
- PMCID: PMC11923758
- DOI: 10.1016/j.clinsp.2025.100583
Pembrolizumab in gestational trophoblastic neoplasia: Systematic review and meta-analysis with sub-group analysis of potential prognostic factors
Abstract
Objective: To assess the performance of pembrolizumab for the treatment of Gestational Trophoblastic Neoplasia (GTN).
Methods: The Medical Subject Headings related to immunotherapy/pembrolizumab and GTN were used alone or in combination to retrieve relevant articles. The authors searched in EMBASE, MEDLINE/PubMed, Elsevier's Scopus, and Web of Science until November/2024. The authors included any randomized controlled trials, cohort studies, case series, and case reports focusing on pembrolizumab treatment in GTN. Meta-analysis of proportions was carried out employing a random-effects model. The meta-analysis employed the inverse variance method, with the arcsine link function for the analysis of proportional data. All analyses were performed using Stata 18. For all analyses, a p-value < 0.05 indicated statistical significance. This study was registered on PROSPERO (CRD42023493329).
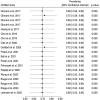
Results: A total of 550 studies were identified after a literature search among which 15 original studies were included in the systematic review and in the meta-analysis. Pembrolizumab induced complete sustained remission in 71.59% (95% CI 53.27‒84.78%; I2 = 0.00%, H2 = 1.00, p = 0.90) of cases. The subgroups meta-analysis showed pembrolizumab had similar performance, regardless of age (< 40 vs. ≥ 40-years-old, p = 0.38), GTN histopathology (Placental Site Trophoblastic Tumor [PSTT], Epithelioid Trophoblastic Tumor [ETT]/noninvasive mole/others versus invasive mole/choriocarcinoma, p = 0.48), time from diagnosis to the beginning of immunotherapy (< 4 vs. ≥ 4-years, p = 0.84), pembrolizumab combined with chemotherapy (yes vs. no, p = 0.66).
Conclusions: Pembrolizumab seems an effective treatment for patients with high-risk GTN with chemoresistant or relapsed disease, including cases of PSTT/ETT, notwithstanding patient age, time to initiate immunotherapy and whether or not it was associated with chemotherapy.
Keywords: Choriocarcinoma; Gestational trophoblastic neoplasia; Immunotherapy; PD-1/PD-L1 inhibitors; Pembrolizumab.
Copyright © 2025. Published by Elsevier España, S.L.U.
Conflict of interest statement
Conflicts of interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Seckl MJ, Sebire NJ, Fisher RA, Golfier F, Massuger L, Sessa C. ESMO Guidelines Working Group. Gestational trophoblastic disease: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 vi39-50. - PubMed
-
- Braga A, Paiva G, Cattai CJ, Elias KM, Horowitz NS, Berkowitz RS. Current chemotherapeutic options for the treatment of gestational trophoblastic disease. Expert Opin Pharmacother. 2023;24(2):245–258. - PubMed
-
- Sharma N, Kundal R, Kaushal V. Immunobiology and immunotherapy of gestational trophoblastic disease. Gynecol Obstet Clin Med. 2022;2(2):76–81.
-
- Abu-Rustum NR, Yashar CM, Bradley K, Brooks R, Campos SM, Chino J, et al. Gestational Trophoblastic Neoplasia, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. Online to NCCN.org. [(accessed on November 4th 2024)]. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1489.
-
- Braga A, Elias KM, Horowitz NS, Berkowitz RS. Treatment of high-risk gestational trophoblastic neoplasia and chemoresistance/relapsed disease. Best Pract Res Clin Obstet Gynaecol. 2021;74:81–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

