Apolipoprotein A-IV is induced by high-fat diets and mediates positive effects on glucose and lipid metabolism
- PMID: 40032158
- PMCID: PMC11938269
- DOI: 10.1016/j.molmet.2025.102119
Apolipoprotein A-IV is induced by high-fat diets and mediates positive effects on glucose and lipid metabolism
Abstract
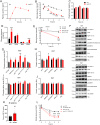
Objective: Low-carbohydrate, high-fat diets under eucaloric conditions are associated with several health-beneficial metabolic effects in humans, particularly in the liver. We recently observed that apolipoprotein A-IV (apoA-IV), a highly abundant apolipoprotein, was among the most upregulated proteins in circulation after six weeks of consuming a high-fat diet in humans. However, the impact of dietary changes in regulating apoA-IV, and the potential effects of apoA-IV on regulation of glucose- and lipid metabolism remain to be fully established.
Methods: We investigated the regulation of circulating fasting concentrations of apoA-IV in humans in response to diets enriched in either fat or carbohydrates. Moreover, to study the whole-body and tissue-specific glucose and lipid metabolic effects of apoA-IV, we administrered apoA-IV recombinant protein to mice and isolated pancreatic islets.
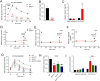
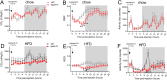
Results: We demonstrate that in healthy human individuals high-fat intake increased fasting plasma apoA-IV concentrations by up to 54%, while high-carbohydrate intake suppressed plasma apoA-IV concentrations. In mice, administration of apoA-IV acutely lowered blood glucose levels both in lean and obese mice. Interestingly, this was related to a dual mechanism, involving both inhibition of hepatic glucose production and increased glucose uptake into white and brown adipose tissues. In addition to an effect on hepatic glucose production, the apoA-IV-induced liver proteome revealed increased capacity for lipoprotein clearance. The effects of apoA-IV in the liver and adipose tissues were concomitant with increased whole-body fatty acid oxidation. Upon glucose stimulation, an improvement in glucose tolerance by apoA-IV administration was related to potentiation of glucose-induced insulin secretion, while apoA-IV inhibited glucagon secretion ex vivo in islets.
Conclusions: We find that apoA-IV is potently increased by intake of fat in humans, and that several beneficial metabolic effects, previously associated with high fat intake in humans, are mimicked by administration of apoA-IV protein to mice.
Keywords: Adipose tissue; Chylomicron; Diet; Fatty acid oxidation; Hepatic glucose production; Incretin hormone; Insulin secretion; Liver.
Copyright © 2025 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: M.L. reports equipment, drugs, or supplies was provided by The University of Sydney. Annemarie Lundsgaard reports a relationship with Novo Nordisk that includes: employment. Jens O. Lagerstedt reports a relationship with Novo Nordisk that includes: employment. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Hansen C.D., Gram-Kampmann E.M., Hansen J.K., Hugger M.B., Madsen B.S., Jensen J.M., et al. Effect of calorie-unrestricted low-carbohydrate, high-fat diet versus high-carbohydrate, low-fat diet on type 2 diabetes and nonalcoholic fatty liver disease : a randomized controlled trial. Ann Intern Med. 2023;176(1):10–21. - PubMed
-
- Skytte M.J., Samkani A., Petersen A.D., Thomsen M.N., Astrup A., Chabanova E., et al. A carbohydrate-reduced high-protein diet improves HbA(1c) and liver fat content in weight stable participants with type 2 diabetes: a randomised controlled trial. Diabetologia. 2019;62(11):2066–2078. - PubMed
-
- Thomsen M.N., Skytte M.J., Astrup A., Deacon C.F., Holst J.J., Madsbad S., et al. The clinical effects of a carbohydrate-reduced high-protein diet on glycaemic variability in metformin-treated patients with type 2 diabetes mellitus: a randomised controlled study. Clin Nutr ESPEN. 2020;39:46–52. - PubMed
-
- London A., Richter M.M., Sjøberg K.A., Wewer Albrechtsen N.J., Považan M., Drici L., et al. The impact of short-term eucaloric low- and high-carbohydrate diets on liver triacylglycerol content in males with overweight and obesity: a randomized crossover study. Am J Clin Nutr. 2024;120(2):283–293. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

