Rhodococcus spp. interacts with human norovirus in clinical samples and impairs its replication on human intestinal enteroids
- PMID: 40032727
- PMCID: PMC11881836
- DOI: 10.1080/19490976.2025.2469716
Rhodococcus spp. interacts with human norovirus in clinical samples and impairs its replication on human intestinal enteroids
Abstract
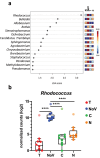
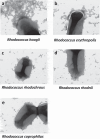
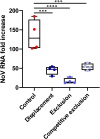
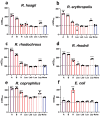
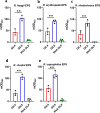
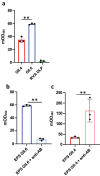
Viral infections, particularly human norovirus (NoV), are a leading cause of diarrheal diseases globally. To better understand NoV susceptibility, it is crucial to investigate both host glycobiology and the influence of the microbiota. Histo-blood group antigens (HBGA) displayed on surfaces of host cells act as NoV receptors, while certain bacteria express HBGA-like substances, facilitating virus-bacteria interactions. To identify bacterial species interacting with NoV during infection, stool samples from children infected with NoV GII.4 were analyzed. The samples were subjected to bacteria separation using anti-NoV GII.4 polyclonal antibodies coupled to magnetic beads, followed by microbiota profiling through 16S rDNA sequencing. This approach identified the genus Rhodococcus as enriched in samples captured with anti-NoV antibodies compared to controls. Electron microscopy confirmed the binding of NoV GII.4 Sydney 2012 viral-like particles (VLP) to five Rhodococcus strains from different species, which expressed HBGA-like substances on their surfaces, particularly from A and B blood groups. In human intestinal enteroids, Rhodococcus erythropolis reduced NoV GII.4 Sydney 2012 infection levels under displacement, exclusion and competitive exclusion settings. Extracellular polysaccharides (EPS) isolated from Rhodococcus strains bound VLP from both GII.4 and GII.6 genotypes. Blocking antibodies targeting A and B epitopes reduced the binding of the EPS from R. erythropolis to GII.6 VLP, while enhanced binding to GII.4 VLP was observed when A and B epitopes were blocked. These findings revealed the interaction of Rhodococcus to NoV in an in vitro setting and open new avenues for developing innovative antiviral strategies to prevent and treat NoV infections through the HBGA-like substances present in their EPS.
Keywords: Human norovirus; Rhodococcus; gut microbiota; histo-blood group antigens; human intestinal enteroids; stool samples.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- World Health Organization (WHO) . The top 10 causes of death [Internet]. 2020. [accessed 2023 June 19]. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
-
- Centers for Disease Control and Prevention (CDC). Norovirus Burden and Trends [Internet]. 2023. [accessed 2023 June 19]. https://www.cdc.gov/norovirus/burden.html#worldwide.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
