Ultra-high dose rate radiotherapy overcomes radioresistance in head and neck squamous cell carcinoma
- PMID: 40032871
- PMCID: PMC11876629
- DOI: 10.1038/s41392-025-02184-0
Ultra-high dose rate radiotherapy overcomes radioresistance in head and neck squamous cell carcinoma
Abstract
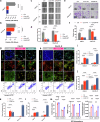
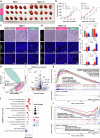
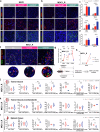
Radiotherapy (RT) resistance in head and neck squamous cell carcinoma (HNSCC) significantly hampers local control and patient prognosis. This study investigated the efficacy and molecular mechanisms of high-energy X-ray-based ultra-high dose rate radiotherapy (UHDR-RT) in overcoming RT resistance. The established RT-resistant HNSCC cell lines and animal models were subjected to UHDR-RT or conventional RT (Conv-RT) via a high-power rhodotron accelerator. Cellular assays assessed the malignant phenotype, viability, and degree of DNA damage, whereas in vivo evaluations focused on tumor proliferation and the tumor immune microenvironment (TiME). Transcriptome sequencing and Olink proteomics were employed to explore the underlying mechanisms involved. In vitro experiments indicated that UHDR-RT suppressed radioresistant cell proliferation and invasion, while promoting apoptosis and exacerbating DNA damage. In contrast, its efficacy in radiosensitive cells was comparable to that of Conv-RT. In vivo studies using patient-derived xenograft nude mice models demonstrated that UHDR-RT only partially reversed RT resistance. Transcriptomic and proteomic analyses of C57BL/6J mice models revealed the predominant role of TiME modulating in reversing radioresistance. Immunofluorescence and flow cytometry confirmed increased CD8+ T cells and an increased M1/M2 macrophage ratio post-UHDR-RT. Mechanistically, UHDR-RT activated CD8+ T cells, which stimulated M1 macrophages through paracrine IFN-γ signaling, thereby enhancing TiME activation. Furthermore, the activated M1 macrophages secreted CXCL9, which in turn reactivated CD8+ T cells, forming a feedforward loop that amplified TiME activation. This study elucidates the dual role of UHDR-RT in directly inducing DNA damage and modulating the TiME, highlighting its potential in treating radioresistant HNSCC.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71, 209–249 (2021). - PubMed
-
- Slater, N. N. et al. Reirradiation in head and neck squamous cell carcinoma; prognostic indicators, oncologic and functional outcomes. Am. J. Otolaryngol.45, 104482 (2024). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

