Knocking down the neuronal lactate transporter MCT2 in the arcuate nucleus of female rats increases food intake and body weight
- PMID: 40032881
- PMCID: PMC11876698
- DOI: 10.1038/s41598-025-90513-2
Knocking down the neuronal lactate transporter MCT2 in the arcuate nucleus of female rats increases food intake and body weight
Abstract
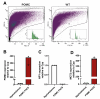
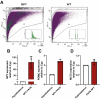
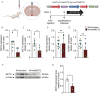
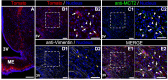
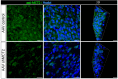
In the arcuate nucleus of the hypothalamus, tanycyte-neuron interactions regulate glucose homeostasis and feeding behavior. Previously, we reported that monocarboxylate transporters (MCT) 1 and 4 are localized in tanycytes, whereas MCT2 is present in arcuate nucleus neurons, including orexigenic and anorexigenic neurons (POMC). MCT1 and MCT4 inhibition impacts feeding behavior, suggesting that monocarboxylate transfer between tanycytes and neurons influences food intake. Electrophysiological studies have shown that POMC neurons respond to lactate through transport and indirect signaling using astrocytic hydroxycarboxylic acid receptor 1. To investigate the role of MCT2 further, we generated MCT2 knockdown rats and analyzed their feeding behavior. Female Sprague-Dawley rats received bilateral injections in the arcuate nucleus with an adeno-associated virus (AAV) carrying a specific short hairpin RNA to inhibit MCT2 expression, thereby generating neuronal MCT2 knockdown rats. Knockdown efficiency in rat hypothalamic tissue was assessed using real-time PCR, Western Blot, and immunohistochemistry. The acute effect on feeding behavior was evaluated following 24 h of fasting, followed by 24 h of refeeding. In MCT2-knockdown rats, we observed additional inhibition of MCT1, suggesting a potential glial response to increased parenchymal lactate levels. Both macrostructure and microstructure of feeding were evaluated in MCT2-knockdown rats and compared to control AAV-injected rats. MCT2 knockdown led to a significant increase in macrostructural parameters, such as food intake and body weight. These findings underscore the importance of lactate transfer as a mechanism in tanycyte-neuron communication mediated by monocarboxylates.
Keywords: Arcuate nucleus; Feeding behavior; MCT2; Rats; Satiation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
-
- Karin Pierre, P. J. M. & Luc Pellerin MCT2 is a Major Neuronal Monocarboxylate Transporter in the Adult Mouse Brain22p. 586–595 (SAGE journals, 2002). 5. - PubMed
-
- Joost, H. G. & Thorens, B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review). Mol. Membr. Biol.18 (4), 247–256 (2001). - PubMed
-
- Garcia-Robles, M. A. et al. Hypothalamic ependymal-glial cells express the glucose transporter GLUT2, a protein involved in glucose sensing. J. Neurochem. 86 (3), 709–724 (2003). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

