Peripheral Biomarker Signatures in Rheumatoid Arthritis-Associated Interstitial Lung Disease
- PMID: 40033001
- PMCID: PMC12311258
- DOI: 10.1002/art.43149
Peripheral Biomarker Signatures in Rheumatoid Arthritis-Associated Interstitial Lung Disease
Abstract
Objective: Rheumatoid arthritis-associated interstitial lung disease (RA-ILD) is a significant cause of morbidity and mortality among patients with RA, yet effective risk stratification for RA-ILD is lacking. We sought to characterize unique peripheral blood biomarker signatures in RA that could improve RA-ILD discrimination beyond clinical and genetic risk factors.
Methods: We performed a cross-sectional study of participants in the Veterans Affairs Rheumatoid Arthritis Registry. ILD was validated through systematic record review. Autoantibodies (n = 14), proinflammatory cytokines/chemokines (n = 36), adipokines (n = 3), alarmins (n = 3), and matrix metalloproteinases (n = 9) were measured from banked serum or plasma. MUC5B rs35705950 genotyping was obtained via microarray. Principal component (PC) analysis was performed to derive unique biomarker signatures. Logistic regression was used to compare models predicting RA-ILD presence using combinations of clinical, peripheral biomarker, and genetic variables.
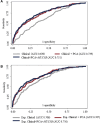
Results: Among 2,001 participants with RA (88.7% male, mean age 63.7 years), 121 (6.4%) had RA-ILD. A total of 15 PCs were identified, with 8 significantly associated with RA-ILD after adjusting for clinical factors. These eight included biomarker themes of innate and allergic responses, autoantibodies (anti-cyclic citrullinated peptide, rheumatoid factor, anti-malondialdehyde-acetaldehyde adduct), adipokines, alarmins, tissue remodeling, and neutrophil chemotaxis. Models with PCs (area under the receiver operating characteristic curve [AUC] 0.739) and PCs with MUC5B (AUC 0.751) outperformed clinical risk factors alone (AUC 0.630; P < 0.001).
Conclusion: Peripheral biomarker signatures are associated with RA-ILD and improve RA-ILD identification beyond clinical risk factors. In addition to demonstrating the potential for peripheral biomarkers to aid RA-ILD risk stratification, these findings suggest diverse pathways are involved in RA-ILD pathogenesis.
© 2025 The Author(s). Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
Figures
References
-
- Marigliano B, Soriano A, Margiotta D, et al. Lung involvement in connective tissue diseases: a comprehensive review and a focus on rheumatoid arthritis. Autoimmun Rev 2013;12(11):1076–1084. - PubMed
-
- Spagnolo P, Lee JS, Sverzellati N, et al. The lung in rheumatoid arthritis: focus on interstitial lung disease. Arthritis Rheumatol 2018;70(10):1544–1554. - PubMed
-
- Chartrand S, Lee JS, Swigris JJ, et al. Clinical characteristics and natural history of autoimmune forms of interstitial lung disease: a single‐center experience. Lung 2019;197(6):709–713. - PubMed
-
- Kelly CA, Saravanan V, Nisar M, et al. Rheumatoid arthritis‐related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics‐‐a large multicentre UK study. Rheumatology (Oxford) 2014;53(9):1676–1682. - PubMed
MeSH terms
Substances
Grants and funding
- 5T32GM075766-17/NH/NIH HHS/United States
- I01 CX001703/CX/CSRD VA/United States
- I01 RX003644/RX/RRD VA/United States
- R01OH012045/OH/NIOSH CDC HHS/United States
- 1U54GM115458/NH/NIH HHS/United States
- I01 BX003635/BX/BLRD VA/United States
- IK2 CX002351/CX/CSRD VA/United States
- T32 GM075766/GM/NIGMS NIH HHS/United States
- RX003644/U.S. Department of Veterans Affairs
- CX001703/U.S. Department of Veterans Affairs
- PR200793/U.S. Department of Defense
- U54 GM115458/GM/NIGMS NIH HHS/United States
- Rheumatology Research Foundation
- IK2 CX002203/CX/CSRD VA/United States
- R01 OH012045/OH/NIOSH CDC HHS/United States
LinkOut - more resources
Full Text Sources
Medical

