Regulation of mammalian cellular metabolism by endogenous cyanide production
- PMID: 40033006
- PMCID: PMC11946912
- DOI: 10.1038/s42255-025-01225-w
Regulation of mammalian cellular metabolism by endogenous cyanide production
Abstract
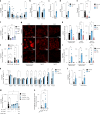
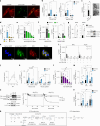
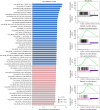
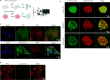
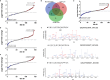
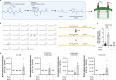
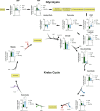
Small, gaseous molecules such as nitric oxide, carbon monoxide and hydrogen sulfide are produced as signalling molecules in mammalian cells. Here, we show that low concentrations of cyanide are generated endogenously in various mammalian tissues and cells. We detect cyanide in several cellular compartments of human cells and in various tissues and the blood of mice. Cyanide production is stimulated by glycine, occurs at the low pH of lysosomes and requires peroxidase activity. When generated at a specific rate, cyanide exerts stimulatory effects on mitochondrial bioenergetics, cell metabolism and cell proliferation, but impairs cellular bioenergetics at high concentrations. Cyanide can modify cysteine residues via protein S-cyanylation, which is detectable basally in cells and mice, and increases in response to glycine. Low-dose cyanide supplementation exhibits cytoprotective effects in hypoxia and reoxygenation models in vitro and in vivo. Conversely, pathologically elevated cyanide production in nonketotic hyperglycinaemia is detrimental to cells. Our findings indicate that cyanide should be considered part of the same group of endogenous mammalian regulatory gasotransmitters as nitric oxide, carbon monoxide and hydrogen sulfide.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






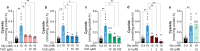


References
-
- Wang, R. Gasotransmitters: growing pains and joys. Trends Biochem. Sci.39, 227–232 (2014). - PubMed
-
- Kelm, M. Nitric oxide metabolism and breakdown. Biochim. Biophys. Acta1411, 273–289 (1999). - PubMed
-
- Moncada, S., Palmer, R. M. & Higgs, E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev.43, 109–142 (1991). - PubMed
-
- Wang, R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol. Rev.92, 791–896 (2012). - PubMed
-
- Wu, L. & Wang, R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol. Rev.57, 585–630 (2005). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

