A western dietary pattern during pregnancy is associated with neurodevelopmental disorders in childhood and adolescence
- PMID: 40033007
- PMCID: PMC12022897
- DOI: 10.1038/s42255-025-01230-z
A western dietary pattern during pregnancy is associated with neurodevelopmental disorders in childhood and adolescence
Abstract
Despite the high prevalence of neurodevelopmental disorders, the influence of maternal diet during pregnancy on child neurodevelopment remains understudied. Here we show that a western dietary pattern during pregnancy is associated with child neurodevelopmental disorders. We analyse self-reported maternal dietary patterns at 24 weeks of pregnancy and clinically evaluated neurodevelopmental disorders at 10 years of age in the COPSAC2010 cohort (n = 508). We find significant associations with attention-deficit hyperactivity disorder (ADHD) and autism diagnoses. We validate the ADHD findings in three large, independent mother-child cohorts (n = 59,725, n = 656 and n = 348) through self-reported dietary modelling, maternal blood metabolomics and foetal blood metabolomics. Metabolome analyses identify 15 mediating metabolites in pregnancy that improve ADHD prediction. Longitudinal blood metabolome analyses, incorporating five time points per cohort in two independent cohorts, reveal that associations between western dietary pattern metabolite scores and neurodevelopmental outcomes are consistently significant in early-mid-pregnancy. These findings highlight the potential for targeted prenatal dietary interventions to prevent neurodevelopmental disorders and emphasise the importance of early intervention.
© 2025. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Ethics statement: The study is conducted in accordance with the Declaration of Helsinki and was approved by the Danish Ethics Committee (H-B-2008-093) and the Danish Data Protection Agency (2015-41-3696). The study is conducted and monitored in accordance with the requirements of GCP as defined in guidelines, EU Clinical Trials Directive (2001/20/EC) and EU GCP Directive (2005/28/EC). All study participants have signed approved informed consent forms before any study-related procedures. The confidentiality of all study participants will be protected in accordance with GCP guidelines. Competing interests: B.E. is part of the Advisory Board of Eli Lilly Denmark A/S, Janssen-Cilag, Lundbeck Pharma A/S, and Takeda Pharmaceutical Company; and has received lecture fees from Bristol-Myers Squibb, Boehringer Ingelheim, Otsuka Pharma Scandinavia AB, Eli Lilly Company and Lundbeck Pharma A/S. B.Y.G. has been the leader of a Lundbeck Foundation Centre of Excellence for Clinical Intervention and Neuropsychiatric Schizophrenia Research (CINS) (January 2009 to December 2021), which was partially financed by an independent grant from the Lundbeck Foundation based on international review and partially financed by the Mental Health Services in the Capital Region of Denmark, the University of Copenhagen and other foundations. All grants are the property of the Mental Health Services in the Capital Region of Denmark and administered by them. She has no other conflicts to disclose. J.L.-S. is a scientific advisor for Precion and a consultant to Tru Diagnostic. All other authors declare no competing interests. The funding agencies did not have any role in design and conduct of the study; collection, management and interpretation of the data; or preparation, review or approval of the paper. No pharmaceutical company was involved in the study.
Figures

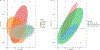

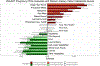







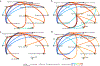


Update of
-
A Western Dietary Pattern during Pregnancy is Associated with Neurodevelopmental Disorders in Childhood and Adolescence.medRxiv [Preprint]. 2024 Jun 25:2024.03.07.24303907. doi: 10.1101/2024.03.07.24303907. medRxiv. 2024. Update in: Nat Metab. 2025 Mar;7(3):586-601. doi: 10.1038/s42255-025-01230-z. PMID: 38496582 Free PMC article. Updated. Preprint.
References
-
- Larsson H, Anckarsater H, Råstam M, Chang Z & Lichtenstein P Childhood attention-deficit hyperactivity disorder as an extreme of a continuous trait: a quantitative genetic study of 8,500 twin pairs. J. Child Psychol. Psychiatry 53, 73–80 (2012). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

