Synthesis, physicochemical characterization and biological activity of novel pyrrole flavones
- PMID: 40033039
- PMCID: PMC11876692
- DOI: 10.1038/s41598-025-91772-9
Synthesis, physicochemical characterization and biological activity of novel pyrrole flavones
Abstract
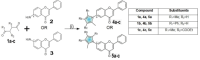
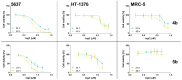
Cancer remains one of the most significant health issues worldwide. By designing compounds with anticancer activity characterized by high selectivity towards cancer cells, medicinal chemistry focuses on the protection of healthy cells and tissues. In this study, we present the hybrid pharmacophore approach, which afforded a series of new pyrrole flavones. The synthetic strategy was based on the Paal-Knorr pyrrole synthesis, starting from aminoflavones through their condensation with 1,4-diketones and leading to 6- and 7-(pyrrol-1-yl) flavones. The isolated products underwent characterization using NMR and UV-VIS spectroscopy, mass spectrometry, TGA, DSC, and Microtox analyses. For all pyrrole flavones, single crystals were obtained and subjected to X-ray diffraction experiments. Their cytotoxic activity was assessed on two human bladder cancer cell lines (5637 and HT-1376) and one non-cancerous (MRC-5) cell line, showing the potential as anticancer agents. Flavone derivative with the 6-(2-methyl-5-phenylpyrrol-1-yl) moiety was active in the MTT assay towards 5637 and HT-1376 cancer cells after 24 h of incubation with IC50 values of 2.97 µM and 5.89 µM, respectively. Notably, flavone derivative with 7-(2-methyl-5-phenylpyrrol-1-yl) revealed cytotoxic activity towards 5637 and HT-1376 cells with IC50 values of 7.39 µM and 13.54 µM, respectively, without any effect on the viability of MRC-5 cells.
Keywords: Anticancer activity; Bladder cancer cell line; Flavonoids; Paal–Knorr synthesis; Pyrrole; X-ray structure.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures







References
-
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov.12, 31–46 (2022). - PubMed
-
- Paduch, R. Theories of cancer origin. Eur. J. Cancer Prev.24, 57–67 (2015). - PubMed
-
- Alseekh, S., Perez De Souza, L., Benina, M. & Fernie, A. R. The style and substance of plant flavonoid decoration; towards defining both structure and function. Phytochemistry174, 112347 (2020). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

