Cholesterol homeostasis and lipid raft dynamics at the basis of tumor-induced immune dysfunction in chronic lymphocytic leukemia
- PMID: 40033083
- PMCID: PMC12041523
- DOI: 10.1038/s41423-025-01262-1
Cholesterol homeostasis and lipid raft dynamics at the basis of tumor-induced immune dysfunction in chronic lymphocytic leukemia
Abstract
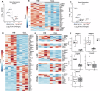
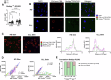
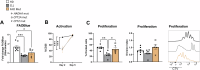
Autologous T-cell therapies show limited efficacy in chronic lymphocytic leukemia (CLL), where acquired immune dysfunction prevails. In CLL, disturbed mitochondrial metabolism has been linked to defective T-cell activation and proliferation. Recent research suggests that lipid metabolism regulates mitochondrial function and differentiation in T cells, yet its role in CLL remains unexplored. This comprehensive study compares T-cell lipid metabolism in CLL patients and healthy donors, revealing critical dependence on exogenous cholesterol for human T-cell expansion following TCR-mediated activation. Using multi-omics and functional assays, we found that T cells present in viably frozen samples of patients with CLL (CLL T cells) showed impaired adaptation to cholesterol deprivation and inadequate upregulation of key lipid metabolism transcription factors. CLL T cells exhibited altered lipid storage, with increased triacylglycerols and decreased cholesterol, and inefficient fatty acid oxidation (FAO). Functional consequences of reduced FAO in T cells were studied using samples from patients with inherent FAO disorders. Reduced FAO was associated with lower T-cell activation but did not affect proliferation. This implicates low cholesterol levels as a primary factor limiting T-cell proliferation in CLL. CLL T cells displayed fewer and less clustered lipid rafts, potentially explaining the impaired immune synapse formation observed in these patients. Our findings highlight significant disruptions in lipid metabolism as drivers of functional deficiencies in CLL T cells, underscoring the pivotal role of cholesterol in T-cell proliferation. This study suggests that modulating cholesterol metabolism could enhance T-cell function in CLL, presenting novel immunotherapeutic approaches to improve outcome in this challenging disease.
Keywords: Cholesterol; Immunotherapy; Leukemia; Lipid metabolism; T-cell.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Niemann CU, Munir T, Moreno C, Owen C, Follows GA, Benjamini O, et al. Fixed-duration ibrutinib–venetoclax versus chlorambucil–obinutuzumab in previously untreated chronic lymphocytic leukaemia (GLOW): 4-year follow-up from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2023;24:1423–33. - PubMed
-
- Khan AN, Asija S, Pendhari J, Purwar R. CAR-T cell therapy in hematological malignancies: Where are we now and where are we heading for? Eur J Haematol. 2024;112:6–18. - PubMed
-
- Siddiqi T, Maloney DG, Kenderian SS, Brander DM, Dorritie K, Soumerai J. et al. Lisocabtagene Maraleucel (liso-cel) in R/R CLL/SLL: 24-month median follow-up of TRANSCEND CLL 004. Blood. 2023;142(Suppl 1):330.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

