Task-sharing and telemedicine delivery of psychotherapy to treat perinatal depression: a pragmatic, noninferiority randomized trial
- PMID: 40033113
- PMCID: PMC12003186
- DOI: 10.1038/s41591-024-03482-w
Task-sharing and telemedicine delivery of psychotherapy to treat perinatal depression: a pragmatic, noninferiority randomized trial
Abstract
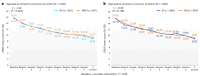
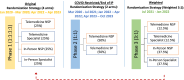
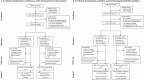
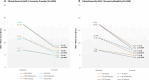
Task-sharing and telemedicine can increase access to effective psychotherapies. Scaling Up Maternal Mental healthcare by Increasing access to Treatment (SUMMIT) is pragmatic, multisite, noninferiority, four-arm trial that tested the non-inferiority of provider (non-specialist vs. specialist providers) and modality (telemedicine vs. in-person) in delivering psychotherapy for perinatal depressive symptoms. Across three university-affiliated networks in the United States and Canada, pregnant and postpartum adult participants were randomized 1:1:1:1 to each arm (472 nonspecialist telemedicine, 145 nonspecialist in-person, 469 specialist telemedicine and 144 specialist in-person) and offered weekly behavioral activation treatment sessions. The primary outcome was depressive symptoms (Edinburgh Postnatal Depression Scale (EPDS)) and the secondary outcome was anxiety (Generalized Anxiety Disorder (GAD-7)) symptoms at 3 months post-randomization. Between 8 January 2020 and 4 October 2023, 1,230 participants were recruited. Noninferiority was met for the primary outcome comparing provider (EPDS: nonspecialist 9.27 (95% CI 8.85-9.70) versus specialist 8.91 (95% CI 8.49-9.33)) and modality (EPDS: telemedicine 9.15 (95% CI 8.79-9.50) versus in-person 8.92 (95% CI 8.39-9.45)) for both intention-to-treat and per protocol analyses. Noninferiority was also met for anxiety symptoms in both comparisons. There were no serious or adverse events related to the trial. This trial suggests compelling evidence for task-sharing and telemedicine to improve access to psychotherapies for perinatal depressive and anxiety symptoms. ClinicalTrials.gov NCT04153864.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: B.N.G. reports compensation for authorship/reviewer for UpToDate chapters on depression. M.L. receives book royalties from Hachette Books and Norton Book. S.M.-B. receives research funding to UNC for clinical trials sponsored by Sage Therapeutics, Electromedical Products International and Sirtsei Pharmaceuticals for clinical trials. She serves as a clinical advisor and professional corporation owner for Modern Health, a digital health company. She also serves as a scientific advisor to EmbarkNeuro and Seaport Therapeutics. B.H.M. holds and receives support from the Labatt Family Chair in Biology of Depression in Late-Life Adults at the University of Toronto. He also receives compensation from the Department of Psychiatry, University of Toronto, Toronto, Ontario; and the Centre for Addiction and Mental Health, Toronto, Ontario. S.N.V. reports royalties from UptoDate Inc. for authorship of materials on depression and pregnancy.
Figures





References
-
- O’hara, M. W. & Swain, A. M. Rates and risk of postpartum depression—a meta-analysis. Int. Rev. Psychiatry8, 37–54 (1996).
-
- Dennis, C.-L., Falah-Hassani, K. & Shiri, R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br. J. Psychiatry210, 315–323 (2017). - PubMed
-
- Howard, L. M. et al. Non-psychotic mental disorders in the perinatal period. Lancet384, 1775–1788 (2014). - PubMed
-
- Curry, S. J. et al. Interventions to prevent perinatal depression: US preventive services task force recommendation statement. JAMA321, 580–587 (2019). - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

