Gastrointestinal toxicity of antibody-drug conjugates: a pharmacovigilance study using the FAERS database
- PMID: 40033454
- PMCID: PMC11874441
- DOI: 10.1186/s40360-025-00877-4
Gastrointestinal toxicity of antibody-drug conjugates: a pharmacovigilance study using the FAERS database
Abstract
Background: Antibody-drug conjugate (ADC) product specifications identify gastrointestinal adverse reactions. Nevertheless, there is a scarcity of comparative studies pertaining to these side effects of similar medications. Special attention is warranted for adverse drug reactions (ADRs) affecting the gastrointestinal system that are inadequately documented in the drug literature.
Aims: Utilizing the U.S. Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS), data mining was conducted to analyze gastrointestinal adverse reactions associated with ADCs. This analysis aimed to provide evidence supporting the safe use of ADCs in medical institutions.
Methods: We utilized the Openvigil 2.1 platform to extract adverse event data reported for each ADC from the FAERS database, covering the period from the drug's launch until the second quarter of 2024. For data analysis, we employed the reporting odds ratio (ROR) and proportional reporting ratio (PRR) methods.
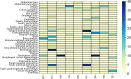
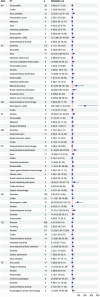
Results: A total of 23,886 adverse event reports were retrieved, with nine ADCs identified as the primary suspected drugs, including 1,517 reports of gastrointestinal adverse events linked to ADCs. The average patient age was 59.69 years, with a higher prevalence of female patients (919 patients, 60.58%) than male patients (319 patients, 24.32%). The gastrointestinal toxicity intensity, ranked from highest to lowest, was as follows: inotuzumab ozogamicin (IO) with ROR = 11.12 and PRR = 10.68, gemtuzumab ozogamicin (GO) with ROR = 7.87 and PRR = 7.54, polatuzumab vedotin (PV) with ROR = 6.47 and PRR = 6.20, brentuximab vedotin (BV) with ROR = 5.79 and PRR = 5.61, sacituzumab govitecan (SG) with ROR = 5.19 and PRR = 4.61, mirvetuximab soravtansine (MS) with ROR = 4.37 and PRR = 3.80, trastuzumab deruxtecan (TD) with ROR = 4.22 and PRR = 3.63, trastuzumab emtansine (TE) with ROR = 3.93 and PRR = 3.85, and enfortumab vedotin (EV) with ROR = 3.26 and PRR = 3.02. Adverse events resulted in 237 deaths, 43 life-threatening cases, and 439 initial or prolonged hospitalizations, with TD being the top ranking for deaths and hospitalizations, followed by TE, which presented the highest mortality rate due to adverse events. The most frequent adverse events were nausea (506 cases), diarrhea (262 cases), vomiting (216 cases), ascites (112 cases), colitis (90 cases), pancreatitis (52 cases), and intestinal obstruction (37 cases).
Conclusions: ADCs may increase the risk of gastrointestinal adverse events and thus require vigilant monitoring in clinical practice.
Keywords: Adverse events (AEs); Antibody–drug conjugate (ADCs); Data mining; FAERS database; Gastrointestinal toxicity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Submission declaration: We affirm the originality of this study, and it has not been submitted to any preprint server. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
A pharmacovigilance study on antibody-drug conjugate (ADC)-related neurotoxicity based on the FDA adverse event reporting system (FAERS).Front Pharmacol. 2024 Feb 7;15:1362484. doi: 10.3389/fphar.2024.1362484. eCollection 2024. Front Pharmacol. 2024. PMID: 38384285 Free PMC article.
-
A pharmacovigilance study on drug-induced liver injury associated with antibody-drug conjugates (ADCs) based on the food and drug administration adverse event reporting system.Expert Opin Drug Saf. 2024 Aug;23(8):1049-1060. doi: 10.1080/14740338.2023.2277801. Epub 2023 Nov 9. Expert Opin Drug Saf. 2024. PMID: 37898875
-
Cardiovascular adverse events associated with antibody-drug conjugates (ADCs): a pharmacovigilance study based on the FAERS database.Front Pharmacol. 2024 May 3;15:1378010. doi: 10.3389/fphar.2024.1378010. eCollection 2024. Front Pharmacol. 2024. PMID: 38766629 Free PMC article.
-
Interstitial lung disease with antibody-drug conjugates: a real-world pharmacovigilance study based on the FAERS database during the period 2014-2023.Ther Adv Respir Dis. 2024 Jan-Dec;18:17534666241299935. doi: 10.1177/17534666241299935. Ther Adv Respir Dis. 2024. PMID: 39660786 Free PMC article.
-
Adverse drug reactions related to methotrexate: a real-world pharmacovigilance study using the FAERS database from 2004 to 2024.Front Immunol. 2025 Jun 4;16:1586361. doi: 10.3389/fimmu.2025.1586361. eCollection 2025. Front Immunol. 2025. PMID: 40534848 Free PMC article. Review.
Cited by
-
Single-Agent and Associated Therapies with Monoclonal Antibodies: What About Follicular Lymphoma?Cancers (Basel). 2025 May 8;17(10):1602. doi: 10.3390/cancers17101602. Cancers (Basel). 2025. PMID: 40427101 Free PMC article. Review.
References
-
- Paolo T, et al. Antibody–drug conjugates: smart chemotherapy delivery across tumor histologies. CA: cancer J Clinicians. 2021;72(2):165–82. - PubMed
-
- Wang J, Yongkun S, Tienan Z, et al. Expert consensus on the clinical application of antibody–drug conjugates for the treatment of malignant tumors (2020 edition). Chin J Med Front (Electronic Edition). 2021;13(1):1–15.
-
- Saber H, Simpson N, Ricks KT, et al. An FDA oncology analysis of toxicities associated with PBD-containing antibody–drug conjugates. Regul Toxicol Pharmacol. 2019;107:104429. - PubMed
-
- Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60190–209. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources