Peripheral Inflammation Profile of Cerebellar Ataxia
- PMID: 40033511
- PMCID: PMC12307985
- DOI: 10.2174/011570159X379620250225075810
Peripheral Inflammation Profile of Cerebellar Ataxia
Abstract
Objectives: The objective of this study is to determine the characteristics of peripheral inflammatory profiles and their correlations with the clinical features in patients with cerebellar ataxia.
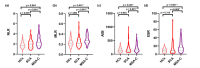
Methods: We conducted a cross-sectional study on a cohort of 140 cerebellar ataxia patients, including 74 patients with spinocerebellar ataxia (SCA), 66 patients with multiple system atrophy with predominant cerebellar ataxia (MSA-C), and 145 healthy controls (HCs). Inflammatory profiles (PLT, MPV, NLR, PLR, MLR, SII, AISI and ESR) were measured in peripheral blood, and were compared by ANOVA and Kruskal-Wallis test. The receiver operating characteristic (ROC) curve and the area under curve (AUC) were performed to determine the sensitivity and specificity of the inflammatory markers. Spearman correlation and partial correlation analysis were performed to detect the association between inflammatory profiles and clinical scales in cerebellar ataxia.
Results: Inflammatory profiles from peripheral blood showed significant difference between different groups. Significant variations were observed in MPV, NLR, MLR, SII, AISI and ESR between cerebellar ataxia and HCs groups (p<0.05). NLR and ESR in both SCA and MSA-C groups were increased compared with HCs (p<0.05). The difference of MHR between SCA and MSA-C groups was observed based on HDL variation (p<0.05). The combination of ESR and PLT distinguished SCA from MSA-C (AUC=0.800). In addition, MLR was significantly corelated with clinical scales, including SARA and ICARS in SCA group as well as UMSARS and FAB in MSA-C group (r>0.3/r<-0.3).
Conclusion: Significant variation in peripheral inflammatory profiles was firstly identified in Chinese genetic ataxias and non-genetic cerebellar ataxia cohort, which showed the potential clinical correlations between peripheral inflammatory phenotype and severity of ataxia.
Keywords: Cerebellar ataxia; RNA.; biomarkers; blood routine examination; peripheral inflammation; receiver operating characteristic (ROC).
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise.
Figures


References
MeSH terms
Substances
Grants and funding
- 2021YFA0805200/National Key R&D Program of China
- 82171254, 82371272, 82301438, 82201411, 82401496/National Natural Science Foundation of China
- 2024JJ3050/Natural Science Foundation of Hunan Province
- R2023047/Major Scientific Research Project for High-level Health Talent in Hunan Province
- 2023SK2084/Furong Lab Research Project
LinkOut - more resources
Full Text Sources
Miscellaneous

