Rejuvenation of Senescent Cells, In Vitro and In Vivo, by Low-Frequency Ultrasound
- PMID: 40033886
- PMCID: PMC12151899
- DOI: 10.1111/acel.70008
Rejuvenation of Senescent Cells, In Vitro and In Vivo, by Low-Frequency Ultrasound
Abstract
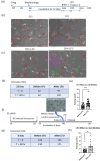
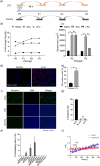
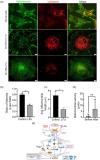
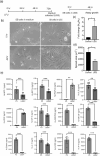
The presence of senescent cells causes age-related pathologies since their removal by genetic or pharmacological means, as well as possibly by exercise, improves outcomes in animal models. An alternative to depleting such cells would be to rejuvenate them to promote their return to a replicative state. Here we report that treatment of non-growing senescent cells with low-frequency ultrasound (LFU) rejuvenates the cells for growth. Notably, there are 15 characteristics of senescent cells that are reversed by LFU, including senescence-associated secretory phenotype (SASP) plus decreased cell and organelle motility. There is also inhibition of β-galactosidase, p21, and p16 expression, telomere length is increased, while nuclear 5mC, H3K9me3, γH2AX, nuclear p53, ROS, and mitoSox levels are all restored to normal levels. Mechanistically, LFU causes Ca2+ entry and increased actin dynamics that precede dramatic increases in autophagy and an inhibition of mTORC1 signaling plus movement of Sirtuin1 from the nucleus to the cytoplasm. Repeated LFU treatments enable the expansion of primary cells and stem cells beyond normal replicative limits without altering phenotype. The rejuvenation process is enhanced by co-treatment with cytochalasin D, rapamycin, or Rho kinase inhibition but is inhibited by blocking Sirtuin1 or Piezo1 activity. Optimized LFU treatment parameters increased mouse lifespan and healthspan. These results indicate that mechanically induced pressure waves alone can reverse senescence and aging effects at the cellular and organismal level, providing a non-pharmacological way to treat the effects of aging.
Keywords: aging; autophagy; calcium signaling; low frequency ultrasound; rejuvenation; senescence.
© 2025 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
Authors (M.S., S.K.K., F.M., B.B.R. and R.M.) are co‐authors of patents related to these studies, and M.S. and F.M. have financial interests in a company, Mechanobiologics Inc. that is planning to market LFU devices suitable for senescent cell rejuvenation in vitro and in vivo.
Figures
References
-
- Alessio, N. , Aprile D., Cappabianca S., Peluso G., Di Bernardo G., and Galderisi U.. 2021. “Different Stages of Quiescence, Senescence, and Cell Stress Identified by Molecular Algorithm Based on the Expression of Ki67, RPS6, and Beta‐Galactosidase Activity.” International Journal of Molecular Sciences 22, no. 6: 3102. 10.3390/ijms22063102. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

