Atherosclerotic plaque instability in symptomatic non-significant carotid stenoses
- PMID: 40034249
- PMCID: PMC11874528
- DOI: 10.1016/j.jvssci.2025.100280
Atherosclerotic plaque instability in symptomatic non-significant carotid stenoses
Abstract
Objective: Carotid endarterectomy for symptomatic carotid stenosis is recommended for patients with >70% stenosis, but not in those with <50%. Because non-significant, low-degree stenoses may still cause strokes, refined risk stratification is necessary, which could be improved by assessing biological features of plaque instability. To challenge risk-stratification based on luminal narrowing, we compared biological features of carotid plaques from symptomatic patients with low-degree (<50%) vs high-degree (>70%) stenosis and explored potential mechanisms behind plaque instability in low-degree stenoses.
Methods: Endarterectomy specimens were taken from symptomatic patients with high-degree (n = 204) and low-degree (n = 34) stenosis, all part of the Biobank of Karolinska Endarterectomies. Patient demographics, image-derived plaque morphology, and gene expression analyses of extracted lesions were used for comparisons. Plaque biology was assessed by transcriptomics using dimensionality reduction, differential gene expression, and gene-set enrichment analyses. Immunohistochemistry was used to study proteins corresponding to upregulated genes.
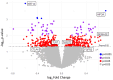
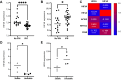
Results: The demographics of the two groups were statistically similar. Calcification, lipid-rich necrotic core, intraplaque hemorrhage, plaque burden, and fibrous cap thickness were similar in both groups, whereas the sum of lipid-rich necrotic core and intraplaque hemorrhage was higher (P = .033) in the high-degree stenosis group. Dimensionality reduction analysis indicated poor clustering separation of plaque gene expression in low-compared with high-degree stenosis lesions, whereas differential gene expression showed upregulation of hypoxia-inducible factor 3A (log2 fold change, 0.7212; P = .0003), and gene-set enrichment analyses identified pathways related to tissue hypoxia and angiogenesis in low-degree stenoses. Hypoxia-inducible factor 3-alpha protein was associated with smooth muscle cells in neo-vascularized plaque regions.
Conclusions: Plaques from symptomatic patients with non-significant low-degree carotid stenoses showed morphologic and biological features of atherosclerotic plaque instability that were comparable to plaques from patients with high-degree stenoses, emphasizing the need for improved stroke risk stratification for intervention in all patients with symptomatic carotid stenosis irrespective of luminal narrowing. An increased expression of hypoxia-inducible factor 3A in low-degree stenotic lesions suggested mechanisms of plaque instability associated with tissue hypoxia and plaque angiogenesis, but the exact role of hypoxia-inducible factor 3A in this process remains to be determined.
Clinical relevance: Carotid plaques from symptomatic patients with <50% stenosis show morphologic and biological features of plaque instability, comparable to high-degree stenosis, which emphasizes the need for improved stroke risk stratification beyond stenosis severity.
Keywords: Atherosclerotic plaque instability; Degree of stenosis; Hypoxia; Stroke risk; Symptomatic carotid stenosis.
© 2025 The Author(s).
Conflict of interest statement
A.B. reports shareholder of Elucid Bioimaging.
Figures






References
-
- Heck D., Jost A. Carotid stenosis, stroke, and carotid artery revascularization. Prog Cardiovasc Dis. 2021;65:49–54. - PubMed
-
- Finn A.V., Nakano M., Narula J., Kolodgie F.D., Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30:1282–1292. - PubMed
-
- Naylor A.R., Rothwell P.M., Bell P.R.F. Overview of the principal results and secondary analyses from the European and North American randomised trials of endarterectomy for symptomatic carotid stenosis. Eur J Vasc Endovasc Surg. 2003;26:115–129. - PubMed
-
- Aboyans V., Ricco J.B., Bartelink M.L.E.L., et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS) Eur J Vasc Endovasc Surg. 2018;55:305–368. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

