Immunological memory to COVID-19 vaccines in immunocompromised and immunocompetent children
- PMID: 40034392
- PMCID: PMC11873107
- DOI: 10.3389/fcimb.2025.1527573
Immunological memory to COVID-19 vaccines in immunocompromised and immunocompetent children
Abstract
Background: Most children in Argentina received only the initial COVID-19 vaccine series, with presumed hybrid immunity after multiple Omicron waves. However, the durability of immune memory, particularly in immunocompromised (IC) children, remains poorly studied.
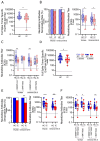
Methods: A cohort of IC (n=45) and healthy children (HC, n=79) was assessed between 13 to 17 months after receiving two or three doses of BBIBP-CorV and/or BNT162b2. Plasma anti-spike IgG, neutralizing activity and antigen-specific CD4+ and CD8+ T cells against Wuhan and Omicron BA.5 variants were assessed.
Results: Most children remained seropositive after two vaccine doses, but compared with HC, IC exhibited lower neutralizing titers against both Wuhan and Omicron BA.5, particularly those vaccinated with BBIBP-CorV. Even after three vaccine doses, IC showed weaker neutralizing antibody response, CD8+ T cell responses and lower IFN-γ production compared with HC. Integrated analysis of neutralizing antibodies, memory CD4+, and CD8+ T cells revealed a weak immune memory among IC with an important compromise in memory CD8+ T cell responses.
Conclusions: Immunity can last up to 17 months, but reduced effectiveness against new variants highlights the need for updated COVID-19 vaccines, especially for IC children. Additional efforts are essential to enhance vaccination coverage and protect this vulnerable population.
Keywords: SARS-CoV-2; T cells; antibodies; children; vaccines; variants.
Copyright © 2025 Russo, Otero, Uranga, Seery, Raiden, Algieri, De Carli, Borda, Albistur, Heinitz, Marcó del Pont, Pardini, Budano, Alvarez, Simaz, Merhar, Quintana, Garbini, Portela, Pereira, Ferrero, Geffner and Arruvito.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures


References
-
- Cotugno N., Franzese E., Angelino G., Amodio D., Romeo E. F., Rea F., et al. . (2022). Evaluation of safety and immunogenicity of bnt162b2 mrna covid-19 vaccine in ibd pediatric population with distinct immune suppressive regimens. Vaccines (Basel) 10 (7), 1109. doi: 10.3390/vaccines10071109 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

