Generation and GMP scale-up of human CAR-T cells using non-viral Sleeping Beauty transposons for B cell malignances
- PMID: 40034423
- PMCID: PMC11874549
- DOI: 10.1016/j.omtm.2025.101425
Generation and GMP scale-up of human CAR-T cells using non-viral Sleeping Beauty transposons for B cell malignances
Abstract
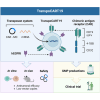
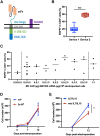
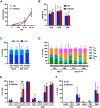
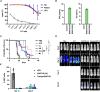
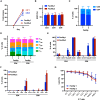
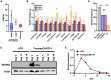
Most CAR-T therapies rely on genetic T cell engineering with integrating viral vectors that, although effective, are associated with prohibitive costs. Here we have generated TranspoCART19 cells, a fully functional 4-1BB second-generation CAR-T cell product targeting CD19, fused to a truncated version of the human EGFR (hEGFRt) as reporter gene and safety switch, based on the Sleeping Beauty transposon delivery system. Our manufacturing protocol allowed generation of TranspoCART19 cells under GMP conditions, showing similar in vitro and in vivo antitumoral efficacy than conventional CAR-T cells generated with lentiviral vectors. Additionally, membrane expression of hEGFRt facilitated in vivo CAR-T cell elimination after cetuximab administration. Safety analyses showed that TranspoCART19 cells presented low vector copy numbers and close-to-random vector integration profiles. Moreover, final TranspoCART19 products lacked non-integrated genomic material used for the generation of CAR-T cells and were free from transposase protein. In vivo biodistribution analyses revealed that TranspoCART19 cells were mainly present in hematopoietic organs with no gender bias. Altogether, this study provides a cost-effective, GMP-compliant manufacturing process for the generation of CAR-T cells using non-viral vectors. These results have supported the approval of a clinical trial to evaluate TranspoCART19 cells in patients with relapsed/refractory lymphoma (NCT06378190) that is currently ongoing.
Keywords: ATMPs; B cell lymphoma; CAR-T cells; Sleeping Beauty transposons.
© 2025 The Authors.
Conflict of interest statement
Z.I. is an inventor on patents related to Sleeping Beauty and MC technology.
Figures

References
-
- Neelapu S.S., Locke F.L., Bartlett N.L., Lekakis L.J., Miklos D.B., Jacobson C.A., Braunschweig I., Oluwole O.O., Siddiqi T., Lin Y., et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/nejmoa1707447. - DOI - PMC - PubMed
-
- Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D., et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. - DOI - PMC - PubMed
-
- Fowler N.H., Dickinson M., Dreyling M., Martinez-Lopez J., Kolstad A., Butler J., Ghosh M., Popplewell L., Chavez J.C., Bachy E., et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat. Med. 2022;28:325–332. doi: 10.1038/s41591-021-01622-0. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

