Sentiment analysis of social media responses to the approval of lecanemab for the treatment of Alzheimer's disease in Japan
- PMID: 40034500
- PMCID: PMC11864249
- DOI: 10.1177/25424823241307639
Sentiment analysis of social media responses to the approval of lecanemab for the treatment of Alzheimer's disease in Japan
Abstract
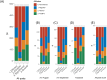
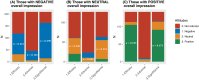
Lecanemab, an anti-amyloid therapy for early Alzheimer's disease, received approval by the FDA and Japan in 2023. Public response on social media was scrutinized, aiming to obtain insights into communication and treatment development. For 478 posts from X and Facebook, their sentiments on efficacy, safety, societal significance, and overall lecanemab impression were assessed by GPT-4 and the authors. Results indicated impressions were 43.7% negative, 26.6% neutral, and 29.7% positive. Social significance concerns dominated negative views. Specific attitude patterns were observed in the overall impression to lecanemab's approval. These insights highlight the need for targeted communication and research on anti-amyloid therapies.
Keywords: Alzheimer's disease; large language model; lecanemab; sentiment analysis; social media.
© The Author(s) 2025.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med 2023; 388: 9–21. - PubMed
-
- Ministry of Health, Labour, and Welfare. Overview of medical service regime in Japan, https://www.mhlw.go.jp/bunya/iryouhoken/iryouhoken01/dl/01_eng.pdf, (2010, accessed 5 November 2023).
-
- Ministry of Finance. Japanese public finance fact sheet, https://www.mof.go.jp/english/policy/budget/budget/fy2022/03.pdf (2022, accessed 5 November 2023).
-
- Eisai. Eisai’s approach to U.S. pricing for Leqembi™ (lecanemab), a treatment for early Alzheimer’s disease, sets forth our concept of “societal value of medicine” in relation to “price of medicine”, https://www.eisai.com/news/2023/news202302.html (2023, accessed 5 November 2023).
LinkOut - more resources
Full Text Sources