Chronic exposure to a synthetic cannabinoid improves cognition and increases locomotor activity in Tg4-42 Alzheimer's disease mice
- PMID: 40034517
- PMCID: PMC11869267
- DOI: 10.1177/25424823241306770
Chronic exposure to a synthetic cannabinoid improves cognition and increases locomotor activity in Tg4-42 Alzheimer's disease mice
Abstract
Background: Alzheimer's disease (AD) is a neurodegenerative disorder characterized by cognitive decline and behavior impairments. Despite recent approvals of anti-amyloid antibodies, there remains a need for disease modifying and easily accessible therapies. Emerging evidence suggests that targeting the endocannabinoid system may hold promise for AD therapy as it plays a crucial role in different physiological processes, including learning, memory and anxiety, as well as inflammatory and immune responses.
Objective: In this study, we investigated the therapeutic potential of the synthetic cannabinoid WIN 55,212-2 on memory deficits in Tg4-42 transgenic mice.
Methods: Tg4-42 mice were assigned to two treatment groups to investigate the preventive effects of WIN 55,212-2 after a prolonged washout period, as well as the therapeutic effects of WIN 55,212-2 on behavior. Furthermore, the effects of WIN 55,212-2 treatment on AD pathology, including inflammation, amyloid-β load, neurogenesis, and brain glucose metabolism, were evaluated.
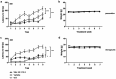
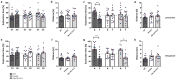
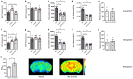
Results: Therapeutic WIN 55,212-2 treatment rescued recognition memory and spatial reference deficits in Tg4-42 mice. Furthermore, therapeutic WIN 55,212-2 administration improved motor performance. In addition, preventative WIN 55,212-2 treatment rescued spatial learning and reference memory deficits. Importantly, WIN 55,212-2 treatment did not affect anxiety-like behavior. However, therapeutic and preventative WIN 55,212-2 treatment resulted in an increase locomotor activity and swimming speed in Tg4-42 mice. WIN-treatment reduced microgliosis in the hippocampus of preventively treated mice and rescued brain glucose metabolism in therapeutically treated Tg4-42 mice.
Conclusions: Our findings emphasize the therapeutic promise of the synthetic cannabinoid WIN 55,212-2 in alleviating behavioral and cognitive deficits linked to AD.
Keywords: Alzheimer's disease; WIN 55,212-2; anxiety; behavior testing; cannabis; medical marijuana.
© The Author(s) 2025.
Conflict of interest statement
The University Medicine Göttingen holds a patent for the Tg4-42 mouse model, with TB being among the inventors. The remaining authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

