Adjuvant endocrine treatment strategies for non-metastatic breast cancer: a network meta-analysis
- PMID: 40034565
- PMCID: PMC11875833
- DOI: 10.1016/j.eclinm.2025.103116
Adjuvant endocrine treatment strategies for non-metastatic breast cancer: a network meta-analysis
Abstract
Background: Multiple trials have evaluated escalation strategies of endocrine therapy for early breast cancer, including ovarian function suppression (OFS) and aromatase inhibitors (AI) in premenopausal patients and extended endocrine therapy. However, several aspects remain controversial due to the heterogeneity of study designs and lack of statistical power in relevant subgroups. We aimed to investigate the optimal endocrine therapy strategy.
Methods: A systematic literature search was performed and last updated in August 2024 to identify randomized controlled trials (RCT) evaluating endocrine treatment strategies for hormone receptor positive breast cancer. A network meta-analysis with a frequentist framework using random-effects model was used to pool direct and indirect evidence. In addition, an extracted individual patient data meta-analysis was conducted to estimate the absolute differences between treatments. Study endpoints were disease-free survival (DFS), overall survival (OS), and safety. PROSPERO: CRD42023447979.
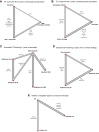
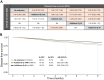
Findings: A total of 37 RCT that had enrolled 107,684 patients were included in the study. During the first five years, OFS + AI was the most effective strategy in premenopausal women, while AI or switch strategy showed the better efficacy results in postmenopausal ones. Following five years of tamoxifen, continuation with five additional years of AI was associated with improved 8-year DFS (85.8%) compared to no extended therapy (78.1%) or five additional years of tamoxifen (81.0%). Following five years of AI or switch strategy, extended treatment with AI improved DFS (Hazard Ratio = 0.81, 95% Confidence Interval 0.73-0.90).
Interpretation: This study provides information regarding the optimal endocrine treatment strategies for patients with resected hormone receptor positive early breast cancer.
Funding: None.
Keywords: Aromatase inhibitor; Breast cancer; Endocrine treatment; Estrogen receptor; Tamoxifen.
© 2025 The Author(s).
Conflict of interest statement
Guillermo Villacampa: speaker's fee from Pfizer, MSD, GSK and Pierre Fabrer; advisory role with AstraZeneca; consultant fees from Reveal Genomics. Mafalda Oliveira: consulting fees from Roche, Seagen, GlaxoSmithKline, Gilead, Puma Biotechnology, AstraZeneca, iTeos Therapeutics, Pierre Fabre, and MSD; research funding from AstraZeneca, Genentech, Roche, Novartis, Immunomedics, Seagen, GlaxoSmithKline, Boehringer Ingelheim, Puma Biotechnology, and Zenith Epigenetics; honoraria from Roche, Seagen, Novartis, AstraZeneca, and Eisai; travel grants from Roche, Pierre Fabre, Novartis, and Eisai; Antonis Valachis: unrestricted research funding paid to institution by Roche and MSD. Tomas Pascual: speaker's fee from Pfizer, AstraZeneca, Novartis, Veracyte and Argenetics; advisory role with Novartis; Alexios Matikas: speaker/consultancy (no personal fees) to Veracyte, Roche, Seagen; research funding paid to institution by MSD, AstraZeneca, Novartis, Veracyte. Andri Papakonstantinou and Victor Navarro have no conflicts of interest to declare.
Figures



References
-
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: a patient-level meta-analysis of 7030 women from four randomised trials. Lancet Oncol. 2022;23(3):382–392. - PMC - PubMed
-
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341–1352. - PubMed
LinkOut - more resources
Full Text Sources

