Immunogenicity and reactogenicity of a booster dose of a typhoid conjugate vaccine (TCV) in Malawian pre-school children
- PMID: 40034570
- PMCID: PMC11872497
- DOI: 10.1016/j.eclinm.2025.103100
Immunogenicity and reactogenicity of a booster dose of a typhoid conjugate vaccine (TCV) in Malawian pre-school children
Abstract
Background: We assessed persistence of typhoid immunity conferred by Vi polysaccharide-tetanus toxoid (Vi-TT) conjugate vaccine (TCV) four years post-vaccination and immunogenicity of a booster dose of Vi-TT given at age five.
Methods: In 2018, a phase 3 trial of Vi-TT in Malawi randomised children 1:1 to receive Vi-TT or meningococcal capsular group A conjugate vaccine (control). Subsequently, TCV was licensed and recommended in the region. In 2023, children vaccinated at 9-11 months in the original trial received a second (Booster- TCV) or first (1st-TCV) Vi-TT dose, at age five. Serum collected at days 0, 28, and 160-180 days after vaccination was tested for anti-Vi immunoglobulin (Ig)G and IgA, reported as enzyme-linked immunosorbent assay units (EU)/mL. Seroconversion was ≥4-fold rise in antibody titers from day 0 to day 28 post-vaccination. Safety outcomes included adverse events during follow-up.
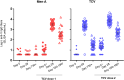
Findings: We enrolled 136 children: 72 Booster-TCV and 64 1st-TCV. At baseline, anti-Vi IgG geometric mean titers (GMT) were higher in Booster-TCV (18.8 EU/mL, 95% CI 15.2-23.2) than 1st-TCV (5.7 EU/mL, 4.6-7.2) arms. GMT increased significantly between days 0 and 28 in both arms, with higher levels in Booster-TCV (6867.9 EU/mL, 5794.1-8140.6) than 1st-TCV (2912.0 EU/mL, 2429.2-3490.7) arms, representing a 375.7 and 492.6 geometric mean fold rise, respectively. On day 28, all Booster-TCV children, and all but one 1st-TCV child, seroconverted. Similar trends were seen for IgA. Vi-TT reactogenicity was similar between vaccine arms.
Interpretation: This study demonstrates sustained Vi-TT immunogenicity four years post-vaccination at 9-11 months old, and robust immune response following a booster dose at five years of age, informing policy decisions on TCV use in children.
Funding: Bill & Melinda Gates Foundation (INV-030857).
Keywords: Africa; Booster dose; Enteric fever; Immunogenicity; Invasive bacterial infection; Malawi; Salmonella enterica; Salmonella typhi; Tetanus; Typhoid conjugate vaccine; Typhoid fever.
Conflict of interest statement
NN, SD, LPJ, and MBL receive funding from the TyVAC grant (INV-030857). KMN received funding from the TyVAC grant (INV-030857) through April 2024. MP's laboratory received funding to support this work from INV-030857. KMN is a WHO Strategic Advisory Group of Experts on Immunisation voting member. The authors reported no additional potential competing interests.
Figures
References
-
- Global Burden of Disease Collaborative Network Global burden of disease study 2021 (GBD 2021) results. 2022. https://vizhub.healthdata.org/gbd-results/
-
- Nampota-Nkomba N., Nyirenda O.M., Khonde L., et al. Safety and immunogenicity of a typhoid conjugate vaccine among children aged 9 months to 12 years in Malawi: a nested substudy of a double-blind, randomised controlled trial. Lancet Glob Health. 2022;10(9):e1326–e1335. doi: 10.1016/S2214-109X(22)00275-3. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

