Predicting tuberculosis drug efficacy in preclinical and clinical models from in vitro data
- PMID: 40034847
- PMCID: PMC11875147
- DOI: 10.1016/j.isci.2025.111932
Predicting tuberculosis drug efficacy in preclinical and clinical models from in vitro data
Abstract
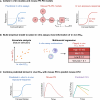
Multiple in vitro potency assays are used to evaluate compounds against Mycobacterium tuberculosis, but a consensus on clinically relevant assays is lacking. We aimed to identify an in vitro assay signature that predicts preclinical efficacy and early clinical outcome. Thirty-one unique in vitro assays were compiled for 10 TB drugs. In vitro EC50 values were compared to pharmacokinetic-pharmacodynamic (PK-PD)-model-derived EC50 values from mice evaluated via multinomial regression. External validation of best-performing in vitro assay combinations was performed using five new TB drugs. Best-performing assay signatures for acute and subacute infections were described by assays that reproduce conditions found in macrophages and foamy macrophages and chronic infection by the ex vivo caseum assay. Subsequent simulated mouse bacterial burden over time using predicted in vivo EC50 was within 2-fold of observations. This study helps us identify clinically relevant assays and prioritize successful drug candidates, saving resources and accelerating clinical success.
Keywords: bioinformatics; biological sciences; natural sciences; pharmacoinformatics; pharmacology.
© 2025 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Global Tuberculosis Report. 2022. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/globa...
-
- Nahid P., Dorman S.E., Alipanah N., Barry P.M., Brozek J.L., Cattamanchi A., Chaisson L.H., Chaisson R.E., Daley C.L., Grzemska M., et al. Executive Summary: Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clin. Infect. Dis. 2016;63:853–867. - PMC - PubMed
-
- Franzblau S.G., Witzig R.S., McLaughlin J.C., Torres P., Madico G., Hernandez A., Degnan M.T., Cook M.B., Quenzer V.K., Ferguson R.M., Gilman R.H. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J. Clin. Microbiol. 1998;36:362–366. - PMC - PubMed

