Identification of a group of 9-amino-acridines that selectively downregulate regulatory T cell functions through FoxP3
- PMID: 40034859
- PMCID: PMC11872463
- DOI: 10.1016/j.isci.2025.111931
Identification of a group of 9-amino-acridines that selectively downregulate regulatory T cell functions through FoxP3
Abstract
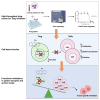
FoxP3+ regulatory T cells (Tregs) are responsible for immune homeostasis by suppressing excessive anti-self-immunity. Tregs facilitate tumor growth by inhibiting anti-tumor immunity. Here, we explored the targeting of FoxP3 as a basis for new immunotherapies. In a high-throughput phenotypic screening of a drug repurposing library using human primary T cells, we identified quinacrine as a FoxP3 downregulator. In silico searches based on the structure of quinacrine, testing of sub-libraries of analogs in vitro, and validation identified a subset of 9-amino-acridines that selectively abrogated Treg suppressive functions. Mechanistically, these acridines interfered with the DNA-binding activity of FoxP3 and inhibited FoxP3-regulated downstream gene regulation. Release from Treg suppression by 9-amino-acridines increased anti-tumor immune responses both in cancer patient samples and in mice in a syngeneic tumor model. Our study highlights the feasibility of screening for small molecular inhibitors of FoxP3 as an approach to pursuing Treg-based immunotherapy.
Keywords: Cancer; Immune response; Immunology.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Liu V.C., Wong L.Y., Jang T., Shah A.H., Park I., Yang X., Zhang Q., Lonning S., Teicher B.A., Lee C. Tumor evasion of the immune system by converting CD4+CD25- T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-beta. J. Immunol. 2007;178:2883–2892. doi: 10.4049/jimmunol.178.5.2883. - DOI - PubMed
LinkOut - more resources
Full Text Sources

