Design of a fragment crystallizable-engineered tetravalent bispecific antibody targeting programmed cell death-1 and vascular endothelial growth factor with cooperative biological effects
- PMID: 40034861
- PMCID: PMC11872405
- DOI: 10.1016/j.isci.2024.111722
Design of a fragment crystallizable-engineered tetravalent bispecific antibody targeting programmed cell death-1 and vascular endothelial growth factor with cooperative biological effects
Abstract
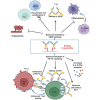
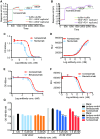
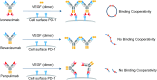
Clinical studies have shown that combination therapy of PD-1 and VEGF antibodies significantly improves clinical benefit over PD-1 antibody alone in certain settings. Ivonescimab, an on-market tetravalent anti-PD-1/VEGF bispecific antibody, was designed to improve efficacy and safety over combo therapy. In this study, the mechanism of action is investigated. In the presence of VEGF, ivonescimab forms soluble complexes with VEGF dimers, leading to the enhanced binding avidity of ivonescimab to PD-1 therefore promoting its increased potency on PD-1/PD-L1-signaling blockade. Likewise, PD-1 binding enhanced ivonescimab binding to VEGF, therefore enhancing VEGF-signaling blockade. Furthermore, ivonescimab treatment demonstrated statistically significant anti-tumor response in vivo. Moreover, ivonescimab contains Fc-silencing mutations abrogating FcγRI/IIIa binding and showed significantly reduced effector function in vitro which is consistent with the better safety profile of ivonescimab in monkeys and humans. Briefly, ivonescimab displays unique cooperative binding and promotes the increased in vitro functional bioactivities with a favorable safety profile.
Keywords: Cancer; Molecular biology; Therapeutics.
© 2025 The Authors.
Conflict of interest statement
No potential conflicts of interest were disclosed by the authors.
Figures





References
-
- Zhou C., Ren S., Luo Y., Wang L., Xiong A., Su C., Zhang Z., Li W., Zhou J., Yu X., et al. A phase Ib/II study of AK112, a PD-1/VEGF bispecific antibody, as first- or second-line therapy for advanced non–small cell lung cancer (NSCLC) J. Clin. Orthod. 2022;40:9040. doi: 10.1200/JCO.2022.40.16_suppl.9040. - DOI
-
- Zhao Y., Chen G., Chen J., Zhuang L., Du Y., Yu Q., Zhuang W., Zhao Y., Zhou M., Zhang W., et al. AK112, a novel PD-1/VEGF bispecific antibody, in combination with chemotherapy in patients with advanced non-small cell lung cancer (NSCLC): an open-label, multicenter, phase II trial. eClinicalMedicine. 2023;62 doi: 10.1016/j.eclinm.2023.102106. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

