Clinicopathological Features and Prognosis of Unresectable Colorectal Cancer With the BRAF V600E Mutation
- PMID: 40034953
- PMCID: PMC11871857
- DOI: 10.21873/cdp.10432
Clinicopathological Features and Prognosis of Unresectable Colorectal Cancer With the BRAF V600E Mutation
Abstract
Background/aim: Patients with unresectable advanced and recurrent colorectal cancer (CRC) and the BRAF V600E mutation have poor prognosis, and conventional chemotherapy is often ineffective. This study aimed to retrospectively evaluate the clinicopathological features and prognosis of this patient population.
Patients and methods: We examined clinicopathological characteristics and treatment outcomes of 26 patients with BRAF V600E-mutated unresectable advanced and recurrent CRC treated between June 2015 and October 2022.
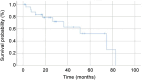
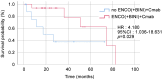
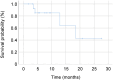
Results: The mean age was 63.1±14.0 years; out of 26 patients, nine (34.6%) were female, 12 (46.2%) had right-sided CRC, and eight (30.8%) had poorly differentiated or mucinous adenocarcinoma. One patient (3.8%) had a RAS mutation, and three (11.5%) had high microsatellite instability. The median overall survival (OS) was 12.0 months. The median OS for patients treated with the BEACON regimen (encorafenib plus cetuximab, with or without binimetinib) was 13.3 months, which was significantly better than that of patients treated without it (7.2 months; hazard ratio=4.180, 95% confidence interval=1.036-18.631, p=0.029). The median progression-free survival for patients treated with BEACON regimen was 6.6 months.
Conclusion: The BRAF V600E mutation was associated with poor prognosis. The BEACON regimen resulted in improved OS compared with other CRC treatment regimens.
Keywords: B-raf; BRAF V600E; Beacon CRC trial; Unresectable advanced recurrent colorectal cancer; binimetinib; encorafenib; mutation; proto-oncogene.
©2025 The Author(s). Published by the International Institute of Anticancer Research.
Conflict of interest statement
The Authors declare no conflicts of interest.
Figures
References
-
- Tabernero J, Grothey A, Van Cutsem E, Yaeger R, Wasan H, Yoshino T, Desai J, Ciardiello F, Loupakis F, Hong YS, Steeghs N, Guren TK, Arkenau HT, Garcia-Alfonso P, Elez E, Gollerkeri A, Maharry K, Christy-Bittel J, Kopetz S. Encorafenib plus cetuximab as a new standard of care for previously treated BRAF V600E-mutant metastatic colorectal cancer: updated survival results and subgroup analyses from the BEACON study. J Clin Oncol. 2021;39(4):273–284. doi: 10.1200/JCO.20.02088. - DOI - PMC - PubMed
-
- Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, Wasan H, Ciardiello F, Loupakis F, Hong YS, Steeghs N, Guren TK, Arkenau HT, Garcia-Alfonso P, Pfeiffer P, Orlov S, Lonardi S, Elez E, Kim TW, Schellens JHM, Guo C, Krishnan A, Dekervel J, Morris V, Calvo Ferrandiz A, Tarpgaard LS, Braun M, Gollerkeri A, Keir C, Maharry K, Pickard M, Christy-Bittel J, Anderson L, Sandor V, Tabernero J. Encorafenib, binimetinib, and cetuximab in BRAF V600E–mutated colorectal cancer. N Engl J Med. 2019;381(17):1632–1643. doi: 10.1056/NEJMoa1908075. - DOI - PubMed
-
- Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kinugasa Y, Murofushi K, Nakajima TE, Oka S, Tanaka T, Taniguchi H, Tsuji A, Uehara K, Ueno H, Yamanaka T, Yamazaki K, Yoshida M, Yoshino T, Itabashi M, Sakamaki K, Sano K, Shimada Y, Tanaka S, Uetake H, Yamaguchi S, Yamaguchi N, Kobayashi H, Matsuda K, Kotake K, Sugihara K, Japanese Society for Cancer of the Colon and Rectum Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25(1):1–42. doi: 10.1007/s10147-019-01485-z. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous