Deubiquitinating Enzymes Regulate Skeletal Muscle Mitochondrial Quality Control and Insulin Sensitivity in Patients With Type 2 Diabetes
- PMID: 40035128
- PMCID: PMC11876994
- DOI: 10.1002/jcsm.13763
Deubiquitinating Enzymes Regulate Skeletal Muscle Mitochondrial Quality Control and Insulin Sensitivity in Patients With Type 2 Diabetes
Abstract
Background: Activation of mitochondrial fission and quality control occur early in the onset of insulin resistance in human skeletal muscle. We hypothesized that differences in mitochondrial dynamics, structure and bioenergetics in humans would explain the onset and progression of type 2 diabetes (T2D).
Methods: Fifty-eight sedentary adults (37 ± 12 years) were enrolled into one of three groups: (1) healthy weight (HW), (2) overweight and obesity (Ow/Ob), or (3) T2D. Body composition, aerobic capacity, and insulin sensitivity were assessed during a 3-day inpatient stay. A fasted skeletal muscle biopsy was obtained to assess mitochondrial functions. C2C12 myoblasts were transfected with FLAG-HA-USP15 and FLAG-HA-USP30 and harvested to assess mitochondrial dynamics and cellular insulin action.
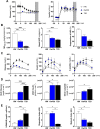
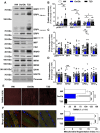
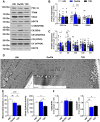
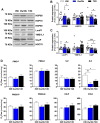
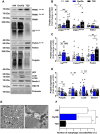
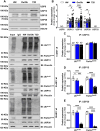
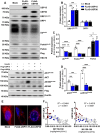
Results: Insulin sensitivity and aerobic capacity were lower in Ow/Ob (132% and 28%, respectively) and T2D (1024% and 83%, respectively) relative to HW. Patients with T2D presented with elevated skeletal muscle mitochondrial fission (3.2 fold relative to HW and Ow/Ob), decreased fusion, and impairments in quality control. Mitochondrial content was lower in Ow/Ob (26%) and T2D (56%). USP13 (84%), USP15 (96%) and USP30 (53%) expression were increased with decreased Parkin and Ub activation in T2D alone. USP15 (R2 = 0.55, p < 0.0001) and USP30 (R2 = 0.40, p < 0.0001) expression negatively correlated with peripheral insulin sensitivity. USP15 and USP30 overexpression activated DRP1 (3.6 and 3.7 fold, respectively) while inhibiting AKT (96% and 81%, respectively) and AS160 (2.1 and 3.5 fold, respectively) phosphorylation.
Conclusion: Mitochondrial fragmentation bypasses defects in mitophagy to sustain skeletal muscle quality control in patients with T2D.
Keywords: bioenergetics; fission; fusion; mitochondria; obesity; quality control; type 2 diabetes.
© 2025 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
Figures
References
-
- Ritov V. B., Menshikova E. V., He J., Ferrell R. E., Goodpaster B. H., and Kelley D. E., “Deficiency of Subsarcolemmal Mitochondria in Obesity and Type 2 Diabetes,” Diabetes 54 (2005): 8–14. - PubMed
-
- Simoneau J. A. and Kelley D. E., “Altered Glycolytic and Oxidative Capacities of Skeletal Muscle Contribute to Insulin Resistance in NIDDM,” Journal of Applied Physiology 83 (1985): 166–171. - PubMed
-
- Mogensen M., Sahlin K., Fernstrom M., et al., “Mitochondrial Respiration Is Decreased in Skeletal Muscle of Patients With Type 2 Diabetes,” Diabetes 56 (2007): 1592–1599. - PubMed
-
- Turner N., Bruce C. R., Beale S. M., et al., “Excess Lipid Availability Increases Mitochondrial Fatty Acid Oxidative Capacity in Muscle: Evidence Against a Role for Reduced Fatty Acid Oxidation in Lipid‐Induced Insulin Resistance in Rodents,” Diabetes 56 (2007): 2085–2092. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

