Photocatalytic Synthesis of Difluorinated Glycoamino Acids and Neoglycopeptides via Hydrodifluoroacetamidation of Vinyl-C-glycosides
- PMID: 40035229
- PMCID: PMC11915378
- DOI: 10.1021/acs.joc.5c00030
Photocatalytic Synthesis of Difluorinated Glycoamino Acids and Neoglycopeptides via Hydrodifluoroacetamidation of Vinyl-C-glycosides
Abstract
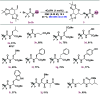
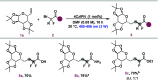
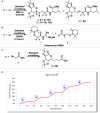
A photocatalytic approach for the synthesis of difluorinated glycoamino acids and neoglycopeptides from bromodifluoroacetamides and sugar-derived olefins is presented. This method stands out because of its simplicity, atomic economy, and mild reaction conditions, allowing compatibility with both natural and unnatural amino acids and peptides. Additionally, it demonstrates efficacy across a variety of carbohydrates, including furanoses, pyranoses, pentose, hexoses, and disaccharides, accommodating an extensive range of protecting groups, even in their deprotected forms.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Visible-Light-Promoted Stereoselective C(sp3 )-H Glycosylation for the Synthesis of C-Glycoamino Acids and C-Glycopeptides.Angew Chem Int Ed Engl. 2022 Jun 13;61(24):e202200822. doi: 10.1002/anie.202200822. Epub 2022 Apr 6. Angew Chem Int Ed Engl. 2022. PMID: 35315966
-
Photoinduced C(sp3)-H Functionalization of Glycine Derivatives: Preparation of Unnatural α-Amino Acids and Late-Stage Modification of Peptides.Acc Chem Res. 2023 Aug 1;56(15):2110-2125. doi: 10.1021/acs.accounts.3c00260. Epub 2023 Jul 19. Acc Chem Res. 2023. PMID: 37467427
-
Non-classical C-saccharide linkage of dehydroalanine: synthesis of C-glycoamino acids and C-glycopeptides.Chem Commun (Camb). 2023 Mar 14;59(22):3305-3308. doi: 10.1039/d2cc06653j. Chem Commun (Camb). 2023. PMID: 36847114
-
The Ramberg-Bäcklund reaction for the synthesis of C-glycosides, C-linked-disaccharides and related compounds.Carbohydr Res. 2006 Jul 24;341(10):1298-311. doi: 10.1016/j.carres.2006.03.013. Epub 2006 May 2. Carbohydr Res. 2006. PMID: 16631146 Review.
-
Synthesis of Selectively gem-Difluorinated Molecules; Chiral gem-Difluorocyclopropanes via Chemo-Enzymatic Reaction and gem-Difluorinated Compounds via Radical Reaction.Chem Rec. 2023 Sep;23(9):e202300028. doi: 10.1002/tcr.202300028. Epub 2023 Mar 22. Chem Rec. 2023. PMID: 36949016 Review.
References
-
- Ferrier R. J.; Hoberg J. O. Synthesis and reactions of unsaturated sugars. Adv. Carbohydr. Chem. 2003, 58, 55–119. 10.1016/S0065-2318(03)58003-9. - DOI - PubMed
- Priebe W.; Fokt I.; Grynkiewicz G.. Glycal Derivatives. In Glycoscience, 2nd ed.; Springer, 2008; pp 699–735.10.1007/978-3-540-30429-6_15 - DOI
-
- Goti G. Catalytic Radical Reactions of Unsaturated Sugars. ChemCatChem 2022, 14, e20220029010.1002/cctc.202200290. - DOI
- Uhrig M. L.; Mora Flores E. W.; Postigo A. Approaches to the Synthesis of Perfluoroalkyl-Modified Carbohydrates and Derivatives: Thiosugars, Iminosugars, and Tetrahydro(thio)pyrans. Chem. - Eur. J. 2021, 27, 7813–7825. 10.1002/chem.202005229. - DOI - PubMed
LinkOut - more resources
Full Text Sources

