A Mutation in the ANK2 Gene Causing ASD and a Review of the Literature
- PMID: 40035441
- PMCID: PMC11877552
- DOI: 10.1002/mgg3.70083
A Mutation in the ANK2 Gene Causing ASD and a Review of the Literature
Abstract
Objective: To investigate the clinical and genetic characteristics of patients with ANK2(HGNC:493)-associated autism spectrum disorders (ASDs) and epilepsy (EP).
Methods: We identified a novel ANK2 variant in a patient with ASD and EP and summarized the clinical and genetic characteristics of ANK2 gene variants in this patient and those in previous reports.
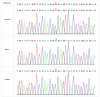
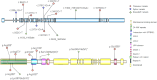
Results: A novel nonsense variant, ANK2 (NM_001148.6):c.3007C>T/p.R1003* in exon 27, was identified in one patient. We described the clinical features and molecular genetics of this patient and previously reported patients. This was discovered at a follow-up visit to the pediatric neurology department where genetic testing based on condition identified this rare genetic variant. He mainly presents with language delay, intellectual disability, limited learning, and communication skills, and later develops seizures, combined with common childhood neurological disorders such as hyperactivity, behavioral abnormalities, and even self-injury. The patient cohort included 16 patients with a complex array of neurological disabilities: ASD (9 patients); EP (10 patients); ASD with EP (4 patients); intellectual disability and developmental delay (5 patients); poor language communication (11 patients); language and learning impairment (11 patients); anxiety/agitation mood disorder (6 patients); attention-deficit/hyperactivity disorder (5 patients); cognitive, memory, and adaptability deficits (1 patient); tic disorder (1 patient); electrocardiogram and cardiac damage (1 patient); and abnormal electroencephalography (EEG) (9 patients).
Conclusion: For the first time, we identified a novel variant of the ANK2 gene in China, broadening the genetic spectrum of the ANK2 gene. ANK2 gene mutations can cause ASD, EP, ASD with EP, developmental delay and intellectual disability, poor language communication skills, language and learning disorders, anxiety/agitation mood disorder, and attention-deficit/hyperactivity disorder. Clinical ASD, EP, common EP should consider the ANK2 gene mutation.
Keywords: ANK2 mutation; ASD; AnkB; EP.
© 2025 The Author(s). Molecular Genetics & Genomic Medicine published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Abdi, K. M. , Mohler P. J., Davis J. Q., and Bennett V.. 2006. “Isoform Specificity of Ankyrin‐b: A Site in the Divergent C‐TERMINAL Domain Is Required for Intramolecular Association.” Journal of Biological Chemistry 281, no. 9: 5741–5749. - PubMed
-
- Bennett, V. , and Stenbuck P. J.. 1979. “The Membrane Attachment Protein for Spectrin Is Associated With Band 3 in Human Erythrocyte Membranes.” Nature 280, no. 5722: 468–473. - PubMed
-
- Fransen, E. , Van Camp G., Vits L., et al. 1997. “L1‐Associated Diseases: Clinical Geneticists Divide, Molecular Geneticists Unite.” Human Molecular Genetics 6, no. 10: 1625–1632. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2015MS08103/Natural Science Foundation of Inner Mongolia Autonomous Region
- YKD2024QN002/Inner Mongolia Medical University youth project
- 2020GG0139/Department of Science and Technology of Inner Mongolia Autonomous Region
- MYYXT201903/Inner Mongolia Autonomous Region Neurological Disease Clinical Medicine Research Center
- kjbw2012005/Major Project of Inner Mongolia Medical University
LinkOut - more resources
Full Text Sources
Medical

