Evidence for peripheral neuroinflammation after acute whiplash
- PMID: 40035629
- PMCID: PMC12444895
- DOI: 10.1097/j.pain.0000000000003560
Evidence for peripheral neuroinflammation after acute whiplash
Abstract
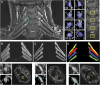
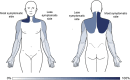
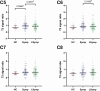
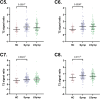
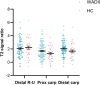
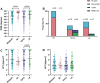
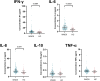
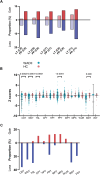
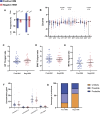
Whiplash injury is associated with high socioeconomic costs and poor prognosis. Most people are classified as having whiplash-associated disorder grade II (WADII), with neck complaints and musculoskeletal signs, in the absence of frank neurological signs. However, evidence suggests that there is a subgroup with underlying nerve involvement in WADII, such as peripheral neuroinflammation. This study aimed to investigate the presence of neuroinflammation in acute WADII using T2-weighted magnetic resonance imaging of the brachial plexus, dorsal root ganglia and median nerve, and clinical surrogates of neuroinflammation: heightened nerve mechanosensitivity (HNM), raised serum inflammatory mediators, and somatosensory hyperalgesia. One hundred twenty-two WADII participants within 4 weeks of whiplash and 43 healthy controls (HCs) were recruited. Magnetic resonance imaging T2 signal ratio was increased in the C5 root of the brachial plexus and the C5-C8 dorsal root ganglia in WADII participants compared with HCs but not in the distal median nerve trunk. Fifty-five percent of WADII participants had signs of HNM. Inflammatory mediators were also raised compared with HCs, and 47% of WADII participants had somatosensory changes on quantitative sensory testing. In those WADII individuals with HNM, there was hyperalgesia to cold and pressure and an increased proportion of neuropathic pain. Many people with WADII had multiple indicators of neuroinflammation. Overall, our results present a complex phenotypic profile for acute WADII and provide evidence suggestive of peripheral neuroinflammation in a subgroup of individuals. The results suggest that there is a need to reconsider the management of people with WADII.
Trial registration: ClinicalTrials.gov NCT04940923.
Keywords: Heightened nerve mechanosensitivity; Magnetic resonance imaging; Neuritis; Neuroinflammation; Neuropathic pain; Quantitative sensory testing; Whiplash; Whiplash associated disorder.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the International Association for the Study of Pain.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures
References
-
- Andersen TE, Hansen M, Ravn SL, Seehuus R, Nielsen M, Vaegter HB. Validation of the PTSD-8 scale in chronic pain patients. Pain Med 2018;19:1365–72. - PubMed
-
- Baumer P, Dombert T, Staub F, Kaestel T, Bartsch AJ, Heiland S, Bendszus M, Pham M. Ulnar neuropathy at the elbow: MR neurography—nerve T2 signal increase and caliber. Radiology 2011;260:199–206. - PubMed
-
- Blankenburg M, Boekens H, Hechler T, Maier C, Krumova E, Scherens A, Magerl W, Aksu F, Zernikow B. Reference values for quantitative sensory testing in children and adolescents: developmental and gender differences of somatosensory perception. PAIN 2010;149:76–88. - PubMed
-
- Boretius S, Gadjanski I, Demmer I, Bahr M, Diem R, Michaelis T, Frahm J. MRI of optic neuritis in a rat model. Neuroimage 2008;41:323–34. - PubMed
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

