Dicer Loss in Müller Glia Leads to a Defined Sequence of Pathological Events Beginning With Cone Dysfunction
- PMID: 40035725
- PMCID: PMC11892533
- DOI: 10.1167/iovs.66.3.7
Dicer Loss in Müller Glia Leads to a Defined Sequence of Pathological Events Beginning With Cone Dysfunction
Abstract
Purpose: The loss of Dicer in Müller glia (MG) results in severe photoreceptor degeneration, as it occurs in retinitis pigmentosa or age-related macular degeneration; however, the sequence of events leading to this severe degenerative state is unknown. The aim of this study was to conduct a chronological functional and structural characterization of the pathological events in MG-specific Dicer-conditional knockout (cKO) mice in vivo and histologically.
Methods: To delete Dicer and mature microRNAs (miRNAs) in MG, two conditional Dicer1 knockout mouse strains (Rlbp-CreER:tdTomato:Dicer-cKOMG and Glast-CreER:tdTomato:Dicer-cKOMG) were created. Optical coherence tomography (OCT), electroretinograms (ERGs), and histological analyses were conducted to investigate structural and functional changes up to 6 months after Dicer deletion.
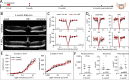
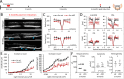
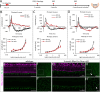
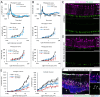
Results: Dicer/miRNA loss in MG leads to (1) impairments of the area spanning from the external limiting membrane (ELM) to the retinal pigment epithelium (RPE), (2) cone photoreceptor dysfunction, and (3) retinal remodeling and functional loss of the inner retina at 1, 3, and 6 months after Dicer loss, respectively, in both of the knockout mouse strains. Furthermore, in the Rlbp-CreER:tdTomato:Dicer-cKOMG strain, rod photoreceptor impairment was found 4 months after Dicer depletion (4) accompanied by alteration of RPE integrity (5).
Conclusions: MG Dicer loss in the adult mouse retina impacts cone function prior to any measurable changes in rod function, suggesting a pivotal role for MG Dicer and miRNAs in supporting cone health. A partially impaired RPE, however, seems to accelerate rod degeneration and overall degenerative events.
Conflict of interest statement
Disclosure:
Figures







Update of
-
Dicer loss in Müller glia leads to a defined sequence of pathological events beginning with cone dysfunction.bioRxiv [Preprint]. 2025 Feb 1:2025.01.30.635744. doi: 10.1101/2025.01.30.635744. bioRxiv. 2025. Update in: Invest Ophthalmol Vis Sci. 2025 Mar 03;66(3):7. doi: 10.1167/iovs.66.3.7. PMID: 39975262 Free PMC article. Updated. Preprint.
References
-
- Barnstable CJ. Epigenetics and degenerative retinal diseases: prospects for new therapeutic approaches. Asia Pac J Ophthalmol (Phila). 2022; 11: 328–334. - PubMed
-
- Sun LF, Chen XJ, Jin ZB.. Emerging roles of non-coding RNAs in retinal diseases: a review. Clin Exp Ophthalmol. 2020; 48: 1085–1101. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

