Butyrate Ameliorates Graves' Orbitopathy Through Regulating Orbital Fibroblast Phenotypes and Gut Microbiota
- PMID: 40035727
- PMCID: PMC11892527
- DOI: 10.1167/iovs.66.3.5
Butyrate Ameliorates Graves' Orbitopathy Through Regulating Orbital Fibroblast Phenotypes and Gut Microbiota
Abstract
Purpose: Graves' orbitopathy (GO), the common extrathyroidal complication of Graves' disease (GD), is characterized by orbital fibroblast stimulation, adipogenesis, and hyaluronan production. Recently, gut microbiota and its metabolites have garnered attention for their possible involvement in GO.
Methods: This study utilized an animal model of GO and examined the effects of butyrate treatment on orbital fibroblast cells and gut microbiota. Ex vivo experiments were performed using orbital fibroblasts derived from healthy patients' and patients' with GO orbital tissue to evaluate vitality, activation, and adipogenesis in response to butyrate treatment. Gut microbiota diversity was also analyzed in butyrate-treated and untreated GO mice.
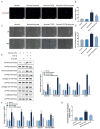
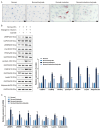
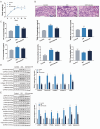
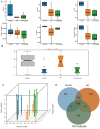
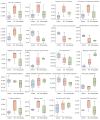
Results: In human orbital fibroblasts, butyrate treatment dramatically decreased the vitality of GO-derived fibroblasts without harming normal fibroblasts. Butyrate prevented activation and fibrotic processes induced by transforming growth factor beta 1 (TGF-β1) in GO and normal fibroblasts. Additionally, butyrate reduced lipid droplet formation and downregulated lipogenic markers in GO and normal orbital fibroblasts, inhibiting adipogenesis. In the GO mouse model, butyrate therapy improved orbital histological abnormalities and normalized serum thyroid hormone and antibody levels. The intestinal microbiome of butyrate-treated GO mice also changed significantly, with a reduction in certain bacteria (Bifidobacterium, GCA-900066575, and Parabacteroides) and an increase in others (Bacteroides and Rikenellaceae_RC9).
Conclusions: Butyrate ameliorates several of the symptoms of GO, lowering GO orbital fibroblast viability, adipogenesis, and TGF-β1-induced fibrosis without damaging normal fibroblasts. Butyrate normalizes thyroid function in a GO mouse model, improves histopathological alterations, and transforms gut microbiota populations, proving its potential in treating GO through the gut-thyroid axis.
Conflict of interest statement
Disclosure:
Figures
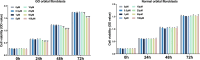





References
-
- Tellez M, Cooper J, Edmonds C.. Graves' ophthalmopathy in relation to cigarette smoking and ethnic origin. Clin Endocrinol (Oxf). 1992; 36(3): 291–294. - PubMed
-
- Wiersinga WM, Bartalena L.. Epidemiology and prevention of Graves' ophthalmopathy. Thyroid. 2002; 12(10): 855–860. - PubMed
-
- Tanda ML, Piantanida E, Liparulo L, et al. .. Prevalence and natural history of Graves' orbitopathy in a large series of patients with newly diagnosed graves' hyperthyroidism seen at a single center. J Clin Endocrinol Metab. 2013; 98(4): 1443–1449. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

