Prodrug activation in malaria parasites mediated by an imported erythrocyte esterase, acylpeptide hydrolase (APEH)
- PMID: 40035755
- PMCID: PMC11912422
- DOI: 10.1073/pnas.2417682122
Prodrug activation in malaria parasites mediated by an imported erythrocyte esterase, acylpeptide hydrolase (APEH)
Abstract
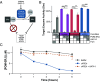
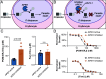
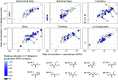
The continued emergence of antimalarial drug resistance highlights the need to develop new antimalarial therapies. Unfortunately, new drug development is often hampered by undesirable drug-like properties of lead compounds. Prodrug approaches temporarily mask undesirable compound features, improving bioavailability and target penetration. We have found that lipophilic diester prodrugs of phosphonic acid antibiotics, such as fosmidomycin (Fsm), exhibit significantly higher antimalarial potency than their parent compounds [R.L. Edwards et al., Sci. Rep. 7, 8400 (2017)]. However, the activating enzymes for these prodrugs were unknown. Here, we show that an erythrocyte enzyme, acylpeptide hydrolase (APEH), is the major activating enzyme of multiple lipophilic ester prodrugs. Surprisingly, this enzyme is taken up by the malaria parasite, Plasmodium falciparum, where it localizes to the parasite cytoplasm and retains enzymatic activity. Using a fluorogenic ester library, we characterize the structure-activity relationship of APEH and compare it to that of P. falciparum esterases. We show that parasite-internalized APEH plays an important role in the activation of substrates with branching at the alpha carbon, in keeping with its exopeptidase activity. Our findings highlight a mechanism for antimicrobial prodrug activation, relying on a host-derived enzyme to yield activation at a microbial target. Mutations in prodrug-activating enzymes are a common mechanism for antimicrobial drug resistance [E. S. Istvan et al., Nat. Commun. 8, 14240 (2017); K. M. V. Sindhe et al., mBio 11, e02640-19 (2020); J. H. Butler et al., Acs Infect Dis. 6, 2994-3003 (2020)]. Leveraging an internalized host enzyme would circumvent this, enabling the design of prodrugs with higher barriers to drug resistance.
Keywords: drug discovery; enzyme; host parasite interactions; malaria; prodrug.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

Update of
-
Prodrug activation in malaria parasites mediated by an imported erythrocyte esterase, acylpeptide hydrolase (APEH).bioRxiv [Preprint]. 2024 Aug 31:2024.08.30.610542. doi: 10.1101/2024.08.30.610542. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2025 Mar 11;122(10):e2417682122. doi: 10.1073/pnas.2417682122. PMID: 39257815 Free PMC article. Updated. Preprint.
References
-
- World Malaria Report 2023 (World Health Organization, 2023).
-
- Balikagala B., et al. , Evidence of artemisinin-resistant malaria in Africa. New Engl. J. Med. 385, 1163–1171 (2021). - PubMed
-
- Artemisinin Resistance and Artemisinin-Based Combination Therapy Efficacy (World Health Organization, 2018).
MeSH terms
Substances
Grants and funding
- R01 AI103280/AI/NIAID NIH HHS/United States
- Paragon of Research Excellence Award/Doris Duke Charitable Foundation (DDCF)
- Senior Research Grant/Indiana Academy of Science (IAS)
- PIDS-St. Jude Children's Research Hospital Fellowship Award in Basic and Translational Science/Pediatric Infectious Diseases Society (PIDS)
- K-Readiness Pilot Grant/Children's Hospital of Philadelphia (CHOP)
- T32AI141393/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01AI123433/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01 AI123433/AI/NIAID NIH HHS/United States
- R01AI171514/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- T32 AI118684/AI/NIAID NIH HHS/United States
- T32 AI141393/AI/NIAID NIH HHS/United States
- R01 AI171514/AI/NIAID NIH HHS/United States
- T32AI118684/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01AI103280/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
LinkOut - more resources
Full Text Sources
Miscellaneous

