Improving IL12 immunotherapy in glioblastoma by targeting the long noncoding RNA INCR1
- PMID: 40035950
- PMCID: PMC12041012
- DOI: 10.1007/s11060-025-04978-2
Improving IL12 immunotherapy in glioblastoma by targeting the long noncoding RNA INCR1
Abstract
Purpose: The potent antitumor effects of interleukin 12 (IL12) gene therapy in glioblastoma (GBM) are significantly attenuated by the highly immunosuppressive microenvironment and the upregulation of the PD-1/PD-L1 immune checkpoint. However, combining IL12 gene therapy with PD-1/PD-L1 inhibitors failed to improve efficacy. This study aims to assess the effects of silencing the immunosuppressive long noncoding RNA INCR1 when combined with IL12 therapy.
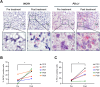
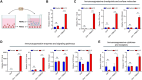
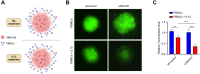
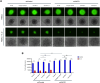
Methods: RNAscope in situ hybridization was performed to analyze INCR1 and PD-L1 expression in tumor tissues from GBM patients pre- and post-IL12 gene therapy. Quantitative PCR was used to analyze immunosuppressive gene expression in patient-derived GBM cells co-cultured with immune cells stimulated with IL12. The effects of INCR1 and PD-L1 silencing on the expression of immunosuppressive genes were evaluated by RNA sequencing. 3D-cytotoxicity assays were performed to assess the activity of immune cells against GBM tumor cells.
Results: INCR1 and PD-L1 expression was upregulated in tumor tissue from GBM patients treated with IL12 gene therapy compared to the tumor tissue of the same patients before the IL12 treatment. Co-culture of patient-derived GBM cells with IL12-stimulated immune cells increased the expression of several immunosuppressive genes. Knocking down INCR1 was more effective than silencing PD-L1 in reducing the expression of multiple immunosuppressive genes. INCR1 silencing improved IL12-mediated immune cell antitumor activity compared to monoclonal antibodies targeting the PD-1/PD-L1 immune checkpoint signaling.
Conclusion: INCR1 silencing affects more immune evasive pathways than PD-L1. Targeting INCR1 may represent a valid approach to improve the efficacy of IL12 therapy in GBM.
Keywords: INCR1; GBM; IL12; Immunotherapy; PD-L1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: Human tissues were analyzed under Brigham and Women’s Hospital/Dana-Farber Cancer Institute Institutional Review Board approval. Consent to participate: Informed consent was obtained from all individual participants included in the study. Consent for publication: Not required. Competing interests: M.M. and E.A.C. are inventors on a patent application covering the use of INCR1 as a therapeutic and diagnostic target. E.A.C. is an advisor to Bionaut Labs, Genenta, inc., Insightec, Inc., Seneca Therapeutics, Theravir. He has equity options in Bionaut Laboratories, Seneca Therapeutics, Ternalys Therapeutics. He is co-founder and on Board of Directors of Ternalys Therapeutics. Patents related to oncolytic viruses are under the possession of Brigham and Women’s Hospital (BWH) with E.A.C. as co-inventors. These patents have been licensed to Candel Therapeutics, Inc. Present and future milestone license fees and future royalty fees are distributed to BWH from Candel. G.J.F. has patents/pending royalties on the PD-L1/PD-1 pathway from Roche, Merck MSD, AstraZeneca, Bristol-Myers-Squibb, Merck KGA, Boehringer-Ingelheim, Dako, Leica, Mayo Clinic, Eli Lilly, and Novartis. G.J.F. has served on advisory boards for iTeos, NextPoint, IgM, GV20, IOME, Bioentre, Santa Ana Bio, Simcere of America, and Geode. G.J.F. has equity in Nextpoint, iTeos, IgM, Invaria, GV20, Bioentre, and Geode.
Figures

References
-
- Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K, Di Meco F, Lieberman F, Zhu JJ, Stragliotto G, Tran D, Brem S, Hottinger A, Kirson ED, Lavy-Shahaf G, Weinberg U, Kim CY, Paek SH, Nicholas G, Bruna J, Hirte H, Weller M, Palti Y, Hegi ME, Ram Z (2017) Effect of Tumor-Treating Fields Plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a Randomized Clinical Trial. JAMA 318:2306–2316. 10.1001/jama.2017.18718 - PMC - PubMed
-
- Hargadon KM, Johnson CE, Williams CJ (2018) Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol 62:29–39. 10.1016/j.intimp.2018.06.001 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

