Boosting effect of high-dose influenza vaccination on innate immunity among elderly
- PMID: 40036077
- PMCID: PMC12016920
- DOI: 10.1172/jci.insight.184128
Boosting effect of high-dose influenza vaccination on innate immunity among elderly
Abstract
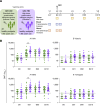
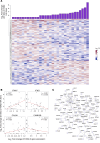
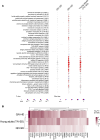
BACKGROUNDThe high-dose quadrivalent influenza vaccine (QIV-HD) showed superior efficacy against laboratory-confirmed illness compared with the standard-dose quadrivalent influenza vaccine (QIV-SD) in randomized controlled trials with the elderly. However, specific underlying mechanism remains unclear.METHODSThis phase IV randomized controlled trial compared early innate responses induced by QIV-HD and QIV-SD in 59 individuals aged > 65 years. Systemic innate cells and gene signatures at day 0 (D0) and D1 as well as hemagglutinin inhibition antibody (HIA) titers at D0 and D21 after vaccination were assessed.RESULTSQIV-HD elicited robust humoral response with significantly higher antibody titers and seroconversion rates than QIV-SD. At D1 after vaccination, QIV-HD recipients showed significant reduction in innate cells, including conventional DCs and NK cells, compared with QIV-SD, correlating with significantly increased HIA titers at D21. Blood transcriptomic analysis revealed greater amplitude of gene expression in the QIV-HD arm, encompassing genes related to innate immune response, IFNs, and antigen processing and presentation, and correlated with humoral responses. Interestingly, comparative analysis with a literature dataset from young adults vaccinated with influenza standard-dose vaccine highlighted strong similarities in gene expression patterns and biological pathways with the elderly vaccinated with QIV-HD.CONCLUSIONQIV-HD induces higher HIA titers than QIV-SD, a youthful boost of the innate gene expression significantly associated with high HIA titers.TRIAL REGISTRATIONEudraCT no. 2021-004573-32.
Keywords: Immunology; Influenza; Innate immunity; Vaccines.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

