Higher dose corticosteroids in hospitalised COVID-19 patients requiring ventilatory support (RECOVERY): a randomised, controlled, open-label, platform trial
- PMID: 40036152
- PMCID: PMC11872607
- DOI: 10.1016/j.eclinm.2025.103080
Higher dose corticosteroids in hospitalised COVID-19 patients requiring ventilatory support (RECOVERY): a randomised, controlled, open-label, platform trial
Abstract
Background: Low dose corticosteroids (e.g., 6 mg dexamethasone) have been shown to reduce mortality for hypoxic COVID-19 patients. We have previously reported that higher dose corticosteroids cause harm in patients with clinical hypoxia but not receiving ventilatory support (the combination of non-invasive mechanical ventilation, including high-flow nasal oxygen, continuous positive airway pressure and bilevel positive airway pressure ventilation, and invasive mechanical ventilation or extra-corporeal membrane oxygenation), but the balance of efficacy and safety in patients receiving ventilatory support is uncertain.
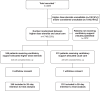
Methods: This randomised, controlled, open-label platform trial (Randomised Evaluation of COVID-19 Therapy [RECOVERY]) assessed multiple possible treatments in patients hospitalised for COVID-19. Eligible and consenting adult patients receiving ventilatory support were randomly allocated (1:1) to either usual care with higher dose corticosteroids (dexamethasone 20 mg once daily for 5 days followed by 10 mg once daily for 5 days or until discharge if sooner) or usual standard of care alone (which includes dexamethasone 6 mg once daily for 10 days or until discharge if sooner). The primary outcome was 28-day mortality; secondary outcomes were duration of hospitalisation and (among participants not on invasive mechanical ventilation at baseline) the composite of invasive mechanical ventilation or death. Recruitment closed on 31 March 2024 when funding for the trial ended. The RECOVERY trial is registered with ISRCTN (50189673) and clinicaltrials.gov (NCT04381936).
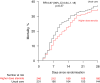
Findings: Between 25 May 2021 and 9 January 2024, 477 COVID-19 patients receiving ventilatory support were randomly allocated to receive usual care plus higher dose corticosteroids vs. usual care alone (of whom 99% received corticosteroids during the follow-up period). Of those randomised, 221 (46%) were in Asia, 245 (51%) in the UK and 11 (2%) in Africa. 143 (30%) had diabetes mellitus. Overall, 86 (35%) of 246 patients allocated to higher dose corticosteroids vs. 86 (37%) of 231 patients allocated to usual care died within 28 days (rate ratio [RR] 0.87; 95% CI 0.64-1.18; p = 0.37). There was no significant difference in the proportion of patients discharged from hospital alive within 28 days (128 [52%] in the higher dose corticosteroids group vs. 120 [52%] in the usual care group; RR 1.04, 0.81-1.33]; p = 0.78). Among those not on invasive mechanical ventilation at baseline, there was no clear reduction in the proportion meeting the composite endpoint of invasive mechanical ventilation or death (76 [37%] of 206 vs. 93 [45%] of 205; RR 0.79 [95% CI 0.63-1.00]; p = 0.05).
Interpretation: In patients hospitalised for COVID-19 receiving ventilatory support, we found no evidence that higher dose corticosteroids reduced the risk of death compared to usual care, which included low dose corticosteroids.
Funding: UK Research and Innovation (Medical Research Council) and National Institute for Health Research (Grant ref: MC_PC_19056), and Wellcome Trust (Grant Ref: 222406/Z/20/Z).
Keywords: COVID-19; Clinical trial; Corticosteroid; Dexamethasone; Mortality.
Crown Copyright © 2025 Published by Elsevier Ltd.
Conflict of interest statement
The authors have no conflict of interest or individual financial relationships relevant to the submitted work to disclose. SF reports institutional support from Pfizer, Sanofi, GSK, J&J, Merck, AstraZeneca, Valneva, Moderna, Novavax, BioNTech and participation on a DMC or advisory board for AstraZeneca, Medimmune, Sanofi, Pfizer, Seqirus, Merck, J&J and MSD. MK reports institutional support from the National Institute for Health and Care Research. WSL reports institutional support from Pfizer and is chair of the UK Joint Committee on Vaccination and Immunisation. MM reports institutional support from Novaris, NovoNordisk and Health Data Research UK and participation on a DMC or steering committee for the National Institute for Health and Care Research. NS reports institutional support from Boehringer-Ingelheim, Eli Lilly and NovoNordisk. No form of payment was given to anyone to produce the manuscript. All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. The Nuffield Department of Population Health at the University of Oxford has a staff policy of not accepting honoraria or consultancy fees directly or indirectly from industry (see https://www.ndph.ox.ac.uk/files/about/ndph-independence-of-research-policy-jun-20.pdf).
Figures

References
-
- UK research and innovation. 13 April 2021 UK-CTAP: record of decisions. 2021. https://www.ukri.org/publications/uk-covid-19-therapeutics-advisory-pane...
Associated data
LinkOut - more resources
Full Text Sources
Medical

