Ongoing Symptoms After Acute SARS-CoV-2 or Influenza Infection in a Case-Ascertained Household Transmission Study: 7 US Sites, 2021-2023
- PMID: 40036243
- PMCID: PMC12159730
- DOI: 10.1093/cid/ciaf026
Ongoing Symptoms After Acute SARS-CoV-2 or Influenza Infection in a Case-Ascertained Household Transmission Study: 7 US Sites, 2021-2023
Abstract
Background: The prevalence and risk factors for ongoing symptoms following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [SCV2]) or influenza infection are not well characterized. We conducted a prospective cohort study of households wherein ≥1 individual was infected with SCV2 or influenza to evaluate prevalence of and factors associated with ongoing symptoms at 90 days.
Methods: Index cases and their household contacts provided baseline health and sociodemographic information and collected daily respiratory specimens for 10 days following enrollment. Participants completed a follow-up survey 90 days after enrollment to characterize ongoing symptoms.
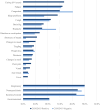
Results: We analyzed 1967 participants enrolled between December 2021 and May 2023. The risk of ongoing symptoms did not differ by infection status in SCV2 (SCV2-positive: 15.6%; SCV2-negative: 13.9%; odds ratio [OR]: 1.14; 95% CI: .7-1.69) or influenza (influenza-positive: 8.8%; influenza-negative: 10.0%; OR: .87; 95% CI: .45-1.72) households. However, among study participants with a documented infection, SCV2-positive participants had nearly twice the odds of ongoing symptoms as influenza-positive participants (OR: 1.92; 95% CI: 1.27-2.97).
Conclusions: These results suggest that SCV2 households have a significantly higher prevalence of ongoing symptoms compared with influenza households (OR: 1.78; 95% CI: 1.28-2.47). Among participants with SCV2 infection, underlying conditions (adjusted OR [aOR]: 2.65; 95% CI: 1.80-3.90) and coronavirus disease 2019 (COVID-19)-like symptoms (aOR: 2.92; 95% CI: 1.15-7.43) during acute infection increased odds of ongoing symptoms at 90 days, whereas hybrid immunity reduced the odds of ongoing symptoms (aOR: 0.44; 95% CI: .22-.90).
Keywords: SARS-CoV-2; hybrid immunity; influenza; long COVID; post-viral symptoms.
Published by Oxford University Press on behalf of Infectious Diseases Society of America 2025.
Conflict of interest statement
Potential conflicts of interest. H. Q. N reports research support from CSL Seqirus, ModernaTX, and GSK outside the submitted work, and served on an advisory board for Moderna and CSL Seqirus. S. R. has received prior grant support from GSK and Biofire. C. G. G has served on an advisory board for Merck and GSK, and received research support from the Centers for Disease Control and Prevention (CDC), National Institutes of Health (NIH), Agency for Healthcare Research and Quality (AHRQ), Food and Drug Administration (FDA), and SyneosHealth. H. K. T has received CDC funding outside the submitted work. M. F.-M. participates on the advisory board for Viiv Healthcare. E. A. is a board member for the Rostropovich Foundation and Fundacion para la Salud Integral de los Guatemaltecos (FUNSALUD), participates on an advisory board for the Curevac and International Vaccine Institute (IVI) Chikungunya vaccine, and has received a research grant from Pfizer paid to his institution. N. B. has received grants from the Doris Duke Clinical Foundation, the North Carolina Seronet Center for Excellence, and University of North Carolina School of Medicine. Y. M. has received a research grant from Pfizer paid to her institution. S. H. M. has received funding from the NIH/National Institute of Child Health and Human Development (NICHD), CDC, and NIH/National Heart, Lung, and Blood Institute (NHLBI) paid to her institution. J. G. P. has received funding from Moderna and CSL Seqirus and has received consulting fees from CSL Seqirus. M. S. S. serves as Associate Director of the American Academy of Pediatrics Pediatric Research in Office Settings. Y. Z. serves on the Data and Safety Monitoring Board (DSMB)/Advisory Board for “Optimizing Psychosocial Treatment for Interstitial Cystitis/Bladder Pain Syndrome” (NIH) and “Comparison of High Dose versus Standard Dose Influenza Vaccines in Lung Allograft Recipients” (National Institute of Allergy and Infectious Diseases [NIAID]). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

