Increased Cerebrospinal Fluid Angiotensin-Converting Enzyme 2 Fragments as a Read-Out of Brain Infection in Patients With COVID-19 Encephalopathy
- PMID: 40036349
- PMCID: PMC12128074
- DOI: 10.1093/infdis/jiaf093
Increased Cerebrospinal Fluid Angiotensin-Converting Enzyme 2 Fragments as a Read-Out of Brain Infection in Patients With COVID-19 Encephalopathy
Abstract
Background: This study assesses the cerebrospinal fluid (CSF) levels of the viral receptor angiotensin-converting enzyme 2 (ACE2) and of the serine protease TMPRSS2 fragments in patients with SARS-CoV-2 infection presenting encephalitis (CoV-Enceph).
Methods: The study included biobanked CSF from 18 CoV-Enceph, 4 subjects with COVID-19 without encephalitis (CoV), 21 with non-COVID-19-related encephalitis (Enceph), and 21 neurologically healthy controls. Participants underwent a standardized assessment for encephalitis. A large subset of samples underwent analysis for an extended panel of CSF neuronal, glial, and inflammatory biomarkers. ACE2 and TMPRSS2 species were determined in the CSF by western blotting.
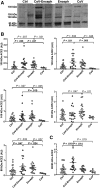
Results: ACE2 was present in CSF as several species, full-length forms and 2 cleaved fragments of 80 and 85 kDa. CoV-Enceph patients displayed increased CSF levels of full-length species, as well as the 80 kDa fragment, but not the alternative 85 kDa fragment, compared with controls and Enceph patients, characterized by increases of both fragments. Furthermore, TMPRSS2 was increased in the CSF of Enceph patients compared with controls, but not in CoV-Enceph patients. The CoV patients without encephalitis displayed unaltered CSF levels of ACE2 and TMPRSS2 species.
Conclusions: Patients with encephalitis displayed an overall increase in CSF ACE2, probably as a consequence of brain inflammation. The increase of the shortest ACE2 fragment only in CoV-Enceph patients may reflect the enhanced cleavage of the receptor triggered by SARS-CoV-2, thus serving to monitor brain penetrance of the virus associated with the rare encephalitis complication. TMPRSS2 changes in the CSF appeared related to inflammation, but not with SARS-CoV-2 infection.
Keywords: ACE2; COVID-19; CSF; TMPRSS2; biomarker.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. H. Z. has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave; has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, and Roche; and is a cofounder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). K. B. has served as a consultant and at advisory boards for Acumen, ALZpath, BioArctic, Biogen, Eisai, Lilly, Moleac, Novartis, Ono Pharma, Prothena, Roche Diagnostics, and Siemens Healthineers; served at data monitoring committees for Julius Clinical and Novartis; given lectures, produced educational materials, and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai, and Roche Diagnostics; and is a cofounder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. M. S. C. has given lectures at symposia sponsored by Almirall, Eli Lilly, Novo Nordisk, Roche Diagnostics, and Roche Farma; received consultancy fees (paid to the institution) from Roche Diagnostics; served on advisory boards of Roche Diagnostics and Grifols; was granted a project and is a site investigator of a clinical trial (funded to the institution) by Roche Diagnostics; and has received in-kind support for research (to the institution) from ADx Neurosciences, Alamar Biosciences, Avid Radiopharmaceuticals, Eli Lilly, Fujirebio, Janssen Research and Development, and Roche Diagnostics. N. J. A. has received consultancy/speaker fees from Bioartic, Biogen, Lilly, Quanterix, and Alamar Biosciences. A. Pa. has received grant support from the Ministry of Health and Ministry of Education, Research and University, and CARIPLO Foundation; and personal compensation as a consultant/scientific advisory board member for Biogen, Lundbeck, Roche, Nutricia, and General Healthcare. A. Pi. has received consultancy/speaker fees from Abbvie, Bial, Lundbeck, Roche, and Zambon pharmaceuticals. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



References
MeSH terms
Substances
Grants and funding
- PI22-01329/Fondo de Investigaciones Sanitarias
- Fondo Europeo de Desarrollo Regional
- Centro de Investigación Biomédica en Red Sobre Enfermedades Neurodegenerativas
- Instituto de Salud Carlos III
- Instituto de Investigación Sanitaria y Biomédica de Alicante
- AICO/2021/308/Direcció General de Ciència i Investigació, Generalitat Valenciana
- INVEST-2023-157/Conselleria d'Innovació, Universitats, Ciència i Societat Digital
- CEX2021-001165-S/Ministerio de Ciencia, Innovación y Universidades, Agencia Estatal de Investigación
- Generalitat Valenciana
- Italian Ministry of University and Research
- ID853981/Horizon 2020
- Ministry of Health
- 2023-00356/Swedish Research Council
- 2022-01018/Swedish Research Council
- 2019-02397/Swedish Research Council
- 2017-00915/Swedish Research Council
- 2022-00732/Swedish Research Council
- 101053962/European Union Horizon 2020
- ALFGBG-71320/Swedish State Support for Clinical Research
- 201809-2016862/Alzheimer Drug Discovery Foundation
- ADSF-21-831376-C/ALZ/Alzheimer's Association/United States
- ADSF-21-831381-C/ALZ/Alzheimer's Association/United States
- ADSF-21-831377-C/ALZ/Alzheimer's Association/United States
- ADSF-24-1284328-C/ALZ/Alzheimer's Association/United States
- SG-23-1038904 QC/ALZ/Alzheimer's Association/United States
- ZEN-21-848495/ALZ/Alzheimer's Association/United States
- Bluefield Project
- Cure Alzheimer's Fund
- Olav Thon Foundation
- Erling-Persson Family Foundation
- Stiftelsen för Gamla Tjänarinnor
- FO2022-0270/Hjärnfonden
- FO2017-0243/Hjärnfonden
- ALZ2022-0006/Hjärnfonden
- European Union Horizon 2020
- Marie Skłodowska-Curie
- 860197/Actions
- JPND2021-00694/European Union
- JPND2019-466-236/European Union
- National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre
- UKDRI-1003/UK Dementia Research Institute at UCL
- Swedish Government and the County
- ALFGBG-715986/Councils
- ALFGBG-965240/Councils
- 2017MYJ5TH/Italian Ministry of University and Research
- 20202THZAW/Italian Ministry of University and Research
- RF-2018-12366209/Italian Ministry of Health
- RF-2019-12369272/Italian Ministry of Health
- PNRR-MAD-2022-12376110/Italian Ministry of Health
- Airalzh
- AGYR2021/Foundation
- LIMPE-DISMOV Foundation
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

