Electrostatic All-Passive Force Clamping of Charged Nanoparticles
- PMID: 40036500
- PMCID: PMC11924585
- DOI: 10.1021/acsnano.4c17299
Electrostatic All-Passive Force Clamping of Charged Nanoparticles
Abstract
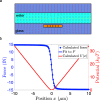
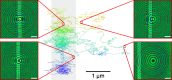
In the past decades, many techniques have been explored for trapping microscopic and nanoscopic objects, but the investigation of nano-objects under arbitrary forces and conditions remains nontrivial. One fundamental case concerns the motion of a particle under a constant force, known as force clamping. Here, we employ metallic nanoribbons embedded in a glass substrate in a capacitor configuration to generate a constant electric field on a charged nanoparticle in a water-filled glass nanochannel. We estimate the force fields from Brownian trajectories over several micrometers and confirm the constant behavior of the forces both numerically and experimentally. Furthermore, we manipulate the diffusion and relaxation times of the nanoparticles by tuning the charge density on the electrode. Our highly compact and controllable setting allows for the trapping and force-clamping of charged nanoparticles in a solution, providing a platform for investigating nanoscopic diffusion phenomena.
Keywords: electrostatic trapping; force clamp; force spectroscopy; gold nanoparticles; nanofluidics; particle tracking; potential mapping.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Howard J.Mechanics of Motor Proteins and the Cytoskeleton; Sinauer Associates Publishers, 2001.
LinkOut - more resources
Full Text Sources
Miscellaneous

