Packaged delivery of CRISPR-Cas9 ribonucleoproteins accelerates genome editing
- PMID: 40036508
- PMCID: PMC11878570
- DOI: 10.1093/nar/gkaf105
Packaged delivery of CRISPR-Cas9 ribonucleoproteins accelerates genome editing
Abstract
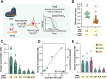
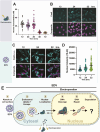
Effective genome editing requires a sufficient dose of CRISPR-Cas9 ribonucleoproteins (RNPs) to enter the target cell while minimizing immune responses, off-target editing, and cytotoxicity. Clinical use of Cas9 RNPs currently entails electroporation into cells ex vivo, but no systematic comparison of this method to packaged RNP delivery has been made. Here we compared two delivery strategies, electroporation and enveloped delivery vehicles (EDVs), to investigate the Cas9 dosage requirements for genome editing. Using fluorescence correlation spectroscopy, we determined that >1300 Cas9 RNPs per nucleus are typically required for productive genome editing. EDV-mediated editing was >30-fold more efficient than electroporation, and editing occurs at least 2-fold faster for EDV delivery at comparable total Cas9 RNP doses. We hypothesize that differences in efficacy between these methods result in part from the increased duration of RNP nuclear residence resulting from EDV delivery. Our results directly compare RNP delivery strategies, showing that packaged delivery could dramatically reduce the amount of CRISPR-Cas9 RNPs required for experimental or clinical genome editing.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nucleic Acids Research.
Conflict of interest statement
The Regents of the University of California have patents issued and pending for CRISPR technologies on which J.D. is an inventor and for delivery technologies on which J.D. and W.N. are co-inventors. J.D. is a cofounder of Azalea Therapeutics, Caribou Biosciences, Editas Medicine, Evercrisp, Scribe Therapeutics, Intellia Therapeutics, and Mammoth Biosciences. J.D. is a scientific advisory board member at Evercrisp, Caribou Biosciences, Scribe Therapeutics, The Column Group and Inari. She also is an advisor for Aditum Bio. J.D. is Chief Science Advisor to Sixth Street, a Director at Johnson & Johnson, Altos and Tempus, and has a research project sponsored by Apple Tree Partners. A.S. has research projects sponsored by Novo Nordisk, Amgen and Merck. All other authors have no competing interests.
Figures



Update of
-
Packaged delivery of CRISPR-Cas9 ribonucleoproteins accelerates genome editing.bioRxiv [Preprint]. 2024 Oct 19:2024.10.18.619117. doi: 10.1101/2024.10.18.619117. bioRxiv. 2024. Update in: Nucleic Acids Res. 2025 Feb 27;53(5):gkaf105. doi: 10.1093/nar/gkaf105. PMID: 39464099 Free PMC article. Updated. Preprint.

