Immuno-Activated, Highly Expressing PD-1 Phenotype in Hepatocellular Carcinoma Is Associated with a Lower Recurrence Rate
- PMID: 40037317
- PMCID: PMC12136520
- DOI: 10.1159/000543933
Immuno-Activated, Highly Expressing PD-1 Phenotype in Hepatocellular Carcinoma Is Associated with a Lower Recurrence Rate
Abstract
Introduction: Curative treatments in early-stage hepatocellular carcinoma (HCC) are limited by high recurrence rates, and targeted therapies against HCC are rare. Tumour microenvironment (TME) plays a crucial role. One strategy is the expression of programmed cell death receptor 1 ligand (PD-L1), whose receptor PD-1 is expressed on activated T cells. The use of immune checkpoint inhibitors (ICIs) has shown antitumour activity. In HCC, ICIs are proposed as a possible first- and second-line therapy. The expression of PD-1 and PD-L1 in the TME is of prognostic importance and can predict response to ICIs. Our study aimed to investigate the impact of intratumoural PD-1 and PD-L1 expression on HCC recurrence and its relation to cancer-immune phenotypes.
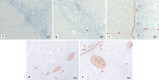
Methods: Immunohistochemical staining was performed on archival tissue from 93 HCC, using the antibodies NAT105 (PD-1) and Ventana SP263 (PD-L1). Tumours were classified as immunologically active, excluded, or deserted. PD-1 and PD-L1 immunoreactivity was evaluated as the proportion of positive immune cells compared to the total immune cells (0%, <1%, and >1%).
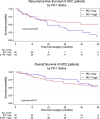
Results: HCC with PD-1 expression in >1% of immune cells had a significantly lower recurrence rate than tumours with <1% PD-1 expression (78%; 38/49, p = 0.027). HCC classified as immune active was also enriched for PD-1 expression >1% (77%; 48/62). Tumours with both characteristics had a significantly lower recurrence rate (p = 0.039).
Conclusion: PD-1 expression on immune cells is associated with a lower recurrence rate in HCC, suggesting a role in HCC recurrence. The therapeutic use of adjuvant anti-PD-1 antibodies should thus be viewed critically and investigated further.
Keywords: Hepatocellular carcinoma; Immune-phenotype; PD-1.
© 2025 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


References
-
- Llovet JM, Villanueva A, Marrero JA, Schwartz M, Meyer T, Galle PR, et al. . Trial design and endpoints in hepatocellular carcinoma: AASLD consensus conference. Hepatology. 2021;73(Suppl 1):158–91. - PubMed
-
- Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. . Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68(2):723–50. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

