A novel long-acting relaxin-2 fusion, AZD3427, improves cardiac performance in non-human primates with cardiac dysfunction
- PMID: 40037346
- PMCID: PMC12160805
- DOI: 10.1093/cvr/cvaf031
A novel long-acting relaxin-2 fusion, AZD3427, improves cardiac performance in non-human primates with cardiac dysfunction
Abstract
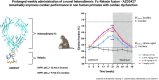
Aims: Relaxin-2, a well-known human hormone primarily associated with pregnancy, has shown promising cardiovascular benefits in both pre-clinical models and clinical trials. However, its therapeutic potential has been limited due to the short half-life and the short duration of treatment. To address this, we developed AZD3427, a novel long-acting relaxin-2 analogue, and assessed its efficacy during prolonged treatment in a large animal model with cardiac dysfunction.
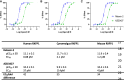
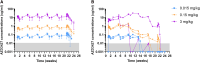
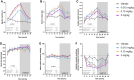
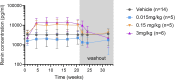
Methods and results: Extensive protein engineering resulted in AZD3427, a novel fusion protein, which closely mimics the natural hormone's structure and consists of a single relaxin-2 and the Fc fragment of human IgG1 to extend its half-life. AZD3427 exhibits an improved pharmacokinetic profile, allowing for weekly or less frequent, subcutaneous dosing, and maintains the pharmacology profile of relaxin-2 with signalling via relaxin family peptide receptor 1 (RXFP1) in cell systems. The effects of chronic RXFP1 agonism with AZD3427 were investigated in a non-human primate (NHP) model with systolic dysfunction and metabolic syndrome. Administration of AZD3427 over a 21-week period led to significant improvements in cardiac function, as evidenced by increased ejection fraction, cardiac output, and stroke volume, as well as reduced systemic vascular resistance. Importantly, no adverse events related to treatments were observed and there were no concomitant changes in heart rate or blood pressure. During the 18-week washout period, the observed effects gradually disappeared.
Conclusion: Prolonged administration of AZD3427, a long-acting relaxin receptor RXFP1 agonist, resulted in remarkable improvement in cardiac function in a NHP model. Findings of this study are an important translational step to developing future therapies and support further clinical development of AZD3427 as a novel treatment for patients with heart failure.
Keywords: AZD3427; Heart failure; Non-human primate; RXFP1; Relaxin-2.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: M.P., M. Antonsson, D.H., K.C., M. Althage, J.O., I.S., J.P., E.M., M.W., A. Sadowska, F.F., N.L., A.B., T.M., S.O., L.J., R.T.G., M.U., D.P., and K.J. are employees of AstraZeneca and may own stock or options. R.T.G. is a former employee and current stockholder of AstraZeneca. He is currently an employee and stockholder of Regeneron Pharmaceuticals. A.G. is a former employee of AstraZeneca and a current employee of Ribocure Pharmaceuticals. A. Shukla is a former employee of AstraZeneca and a current employee of Merck. S.K. is currently a PhD student at University of Cambridge, funded by AstraZeneca. P.C. is a former employee of AstraZeneca and a current employee of Apconix. W.H. is a former employee of AstraZeneca and a current employee of Experimental Drug Development Centre (EDDC), A*STAR, Singapore. R.P. is an employee of KBI.
Figures

Comment in
-
Revivifying research on relaxin receptor-targeted therapy for cardiovascular diseases.Cardiovasc Res. 2025 Jun 12;121(6):836-838. doi: 10.1093/cvr/cvaf057. Cardiovasc Res. 2025. PMID: 40241319 No abstract available.
References
-
- Lippi G, Sanchis-Gomar F. Global epidemiology and future trends of heart failure. AME Med J 2020;5:15–15.
-
- Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014;63:1123–1133. - PubMed
-
- McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, Bohm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukat A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjostrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. - PubMed
-
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. - PubMed
-
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Skibelund AK; ESC Scientific Document Group . 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2023;44:3627–3639.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

